1. Background
The science of tissue culture has been widely developed to help create a large number of plants in a relatively short period. For the growth of in vitro plants, there is a need for a culture medium containing mineral salts. Furthermore, the culture of plant tissues and cells requires a variety of compounds, including plant hormones (phytohormones), vitamins, and sucrose (1, 2).
Unfortunately, such a culture medium, rich in nutrients, can also provide the basis for the rapid growth of bacteria and fungi. This type of contamination in the culture medium usually develops rapidly and negatively affects plant growth and ultimately destroys the cultured plant tissue by evacuating nutrients in the culture medium and producing toxic substances. Therefore, the complete disinfection of food culture medium, containers, seeds, tissues, and any means used in culture is unavoidable by heat or filtration in this standard tissue culture system (3-6). Furthermore, any procedure for tissue culture should be performed in the purified air stream under the laminar hood. Despite using these precise measures to maintain sterile conditions, the contamination of cultivated plants can be considered a serious problem that sometimes destroys some plants and even all plants cultivated (6, 7).
Rhazya stricta belongs to the Apocynaceae family and Rauwolfioideae subfamily. It is burned like Esfand in Saudi Arabia. Rhazya stricta is a small evergreen plant with leathery, thick, long, oval, and narrow leaves on both sides. The average length of the leaf is 7 - 10 cm, with a width of 12 cm in the middle. The flowers are white and slightly fragrant. The fruit is 6 cm long and 0.5 cm wide; long seeds appear 1 cm in length. This plant is distributed in India, Afghanistan, Saudi Arabia, and Iran. It is found in the forests of the southeastern subtropical region between Chabahar, Nikshahr, Iranshahr, and Saravan in Iran. This plant has been used as a medicine to treat various diseases in Afghanistan, India, Iran, Pakistan, Qatar, and Saudi Arabia. It has more than 50 different Indole alkaloids, some of which have anti-cancer properties, such as valesiacotamine and tetrahydrozoline. The raw ethanolic extract of R. stricta fruit has antimicrobial activity (8, 9).
Methanolic and chloroformic extracts of roots have antimicrobial and antifungal activity against E. coli, Aspergillus flavus, A. terreus, B. subtilis, S. aureus, and C. albicans. Aqueous extract of R. stricta has antimicrobial activity against Neisseria meningitides (10-16). Rhazya stricta and its metabolites help cure cancer, skin diseases, hypertension, rheumatism, sore throat, syphilis, and fever (17). Various studies have found that different parts of R. stricta contain many phytochemical elements such as alkaloids, flavonoids, and terpenes (18). Some natural substances in plants have antimicrobial properties and are used as a seasoning in foods in some countries. The vegetable aromatics are used in foods not only for their aroma but also for their food preservation and medicinal properties (19-23).
Different types of antimicrobial chemicals have been used in plant tissue culture. Antibiotics have been extensively tested to prevent or reduce the growth of bacteria in tissue culture (3-5, 24). However, antibiotics are less applied because they are expensive and only work against bacteria. Furthermore, they are less efficacious against various bacteria, are usually unstable, and often produce phytotoxic properties (25-27).
A study (6) reported a method for removing fungal contamination in cyclamen tissue culture that involved immersing cyclamen tissue fragments in imidazole and triazole fungicidal solution or culturing a fragment of cyclamen tissue in the culture medium containing this fungicide. It examined the microbial contamination and its sources in tissue cultures of cassava, hemp, cowpea, and banana in Nigeria. The results indicated that the emergence of bacterial infection was faster than fungal infection in plant tissue culture. Nineteen infectious agents, including 11 bacterial and eight fungal species, were found in tissue culture media. Bacterial infections included Pseudomonas fluorescens, Escherichia coli, Proteus species, Micrococcus species, Streptococcus pneumoniae, Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Corynebacterium species, and Ironenia species, and extracted fungi included Alternaria tenuis, Aspergillus niger, Aspergillus fumigatus, Cladosporium species, Saccharomyces, Fusarium oxysporum, Rhizopus nigracans, and Fusarium culmorum (28-30). The results indicated that plant explants' most transmitted infections included P. fluorescens, Corynebacterium species, B. subtilis, A. niger, R. nigracans, and F. camrom (25 - 39%). The user-transmitted infection included B. cereus, E. coli, and S. aureus (48 - 36%), the workplace-borne contaminants included E. coli (12%) and S. aureus (16%), and ambient airborne contamination included Bacillus, Streptococcus faecalis, and E. coli. Glove-borne infections included Corynebacterium, B. subtilis, S. pneumoniae, and S. faecalis.
Date palm with the scientific name of Phoenix dactylifera L. is probably a Phoenician word. This is a monocotyledonous plant of the Palmaceae family, diploid (2n = 36), perennial, evergreen, dioecious, and heterozygous, with a meager growth rate. It is native to tropical and subtropical regions of Africa and South Asia (31, 32).
Dates are essential export products of Iran, and their export has a long history. In Iran, Khuzestan, Hormozgan, Kerman, Fars, Sistan and Baluchestan, and Bushehr provinces produce 99% of Iranian dates. The medicinal importance of dates has been proven with the advancement of medical and nutritional knowledge. Dates contain calcium, which is a major factor in bone strength. It also contains a significant amount of phosphorus, a vital element for the brain (33-35).
It was found that the maximum contamination in palm tissue culture was due to Aspergillus niger, Penicillium sp., and Alternaria alternata with rates of 16.8%, 10%, and 8%, and the minimum contamination was caused by Trichoderma harzianum and Nigrospora sp. (36).
Two major groups of contaminants were identified in date tissue culture laboratories in Iraq. The first group included fungi, the most predominant of which were A. niger and Alternaria alternate, and the second group included bacteria belonging to Bacillus and Staphylococcus genera (37).
Actinomycin at concentrations of 0, 100, 250, 500, and 1000 mg/L has been used at different stages of callus production, body embryogenesis, branching, and rooting of palm tissue culture to control the contamination of date palm explants. The results indicated that a concentration of 500 mg/L of actinomycin led to contamination-free culture (38).
2. Objectives
This experiment aimed to investigate the antifungal and antibacterial properties of R. stricta essential oil and extract in Murashige and Skoog culture media and their effects on the growth of date plants.
3. Methods
To investigate the antifungal and antibacterial properties of R. stricta extract in Murashige and Skoog media and their effects on date growth, we conducted a factorial experiment as a completely randomized design with three replications in 2021 at the tissue culture laboratory of the Agricultural Biotechnology Research Institute in University of Zabol.
3.1. Preparation of Aqueous Extract of Rhazya stricta
We soaked 20 g of powdered plant leaves separately in 80% distilled water and ethanol solvents and kept them on a shaker for 24 hours. After one day, the material was filtered with Whatman grade-2 filter paper. A vacuum rotary apparatus removed the solvents from the filtered material. The concentrated extracts were stored in an oven at 40°C for 48 hours until a pure extract was obtained and the solvent was removed entirely. Finally, the obtained extracts were dried and kept in the refrigerator at 4°C until the test after weighing.
3.2. Preparation of Plant Samples
The plant materials used in this experiment included date offshoots prepared from Iranshahr groves (Sistan and Baluchestan Province) and transferred to the laboratory (Figure 1A). Palm roughages were cut and removed using a saw and knife by a longitudinal cut in the sheath (Figure 1B).
3.3. Preparation of Aqueous Extract of Rhazya stricta
We soaked 20 g of powdered plant leaves and roots separately in distilled water solvent and kept them on a shaker for 24 hours. After one day, the material was filtered with Whatman grade-2 filter paper. The concentrated extracts were stored in an oven at 40°C for 48 hours. Finally, the extracts were dried and kept in the refrigerator at 4°C until the test after weighing.
3.4. Preparation of Ethanolic Extract of Rhazya stricta
We soaked 20 g of powdered plant leaves and roots separately in 80% ethanol and kept them on a shaker for 24 hours. After one day, the material was filtered with Whatman grade-2 filter paper. A vacuum rotary apparatus removed the solvents from the filtered material. The concentrated extracts were stored in an oven at 40°C for 48 hours until a pure extract was obtained and the solvent was removed entirely. Finally, the extracts were dried and kept in the refrigerator at 4°C until the test after weighing.
3.5. Culture of Explants
First, Murashige and Skoog (MS) culture medium containing Benzyl Adenine (BA) (0.5 mg/L), 3% sucrose, 9 g/L agar, and pH 5.7 was prepared, and then the R. stricta extract was prepared from the aerial organs and root at concentrations of 25, 50, and 70 ppm under the laminar hood. Before freezing, the substrate was added to the culture medium by a 0.45-µ syringe filter. The substrate without plant extract was considered as a control.
3.6. Disinfection of Explants
The explants were washed with dishwashing liquid for disinfection and then rinsed with running water. The samples were then sterilized with 3% sodium hypochlorite for 20 minutes. Of course, sodium hypochlorite was tested at different concentrations, but 3% was better, as high concentrations caused burns and tissue loss. Finally, they were rinsed three times with sterile distilled water and transferred to the culture medium under an isolated laminar hood.
3.7. Storage Conditions of Samples
Before treatments, the samples were stored in a 37°C incubator for three weeks to determine the contamination percentage. Three samples were cultured for each alcoholic and aqueous extract at each concentration, i.e., three replicates per concentration. Samples were stored at 20 ± 2°C. Photoperiodic lighting (16 hours of light and 8 hours of dark) and the percentage growth inhibition of bacteria and fungi were measured after one month.
3.8. Data Analysis
The factorial experiment was conducted as a completely randomized design with three replicates. Data analysis was done using SAS software, and the GLM test with a probability level of 5% was used for the mean comparison test. All diagrams were drawn using Excel software.
4. Results
According to Table 1, the simple effects of organ type (leaf or root), type of extract (ethanolic or aqueous), and extract concentration (0, 25, 50, and 75 ppm) on the percentage of contamination of date explants were significant in the culture medium at 5% probability level. The study of dual interactions indicated that the interaction of plant organs×extract was significant at a probability level of 1%, and the dual interaction type of extract×concentration was significant at a probability level of 5%. However, the interaction of organs× extract concentration was not significant. The interaction of the three factors in the test was significant at a probability level of 1% (Table 1).
| Sources of Changes | Degree of Freedom | Contamination Percentage |
|---|---|---|
| Replication (r) | 2 | 258.33 ns |
| Organ (a) | 1 | 4408.33* |
| Extract type (b) | 1 | 8008.33* |
| Concentration (c) | 3 | 7275* |
| Organ × extract | 1 | 675** |
| Organ × extract concentration | 3 | 208.33 ns |
| Extract type × concentration | 3 | 1408.33* |
| Organ × extract type × concentration | 3 | 341.66** |
| Test error | - | 89.44 |
| Coefficient of variation | - | 16.56 |
a * Significant at a 5% probability level; ** Significant at a 1% probability level; ns, non-significant.
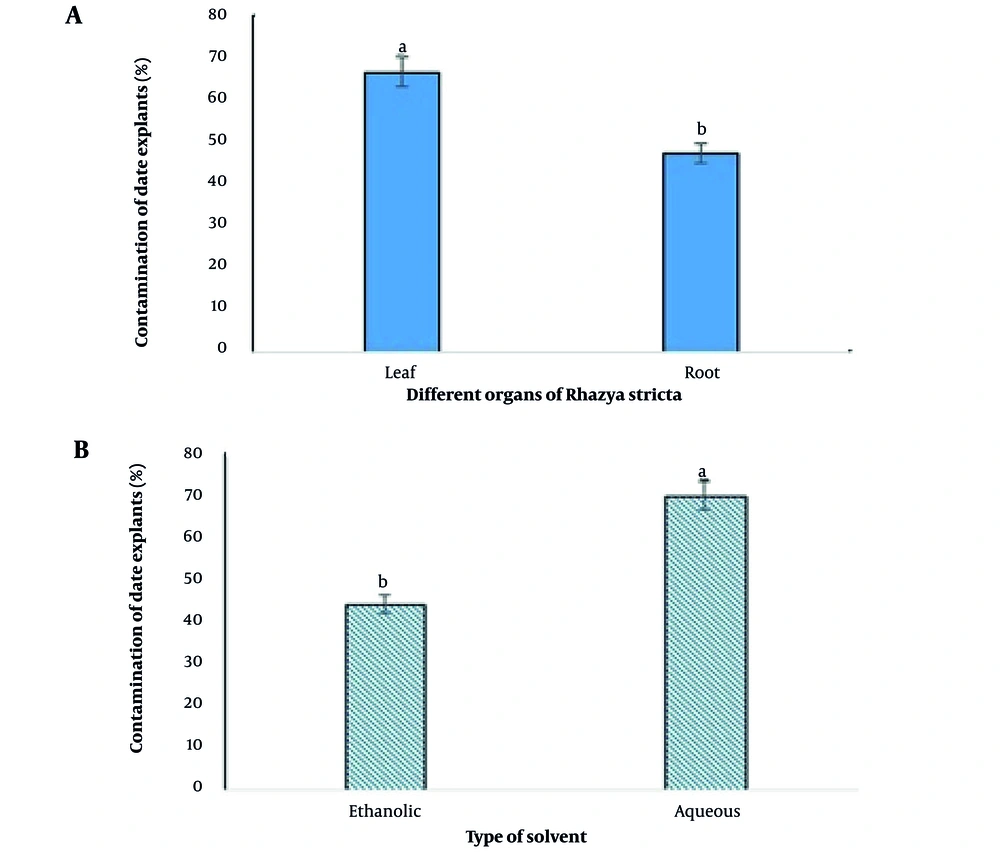
As shown in Figure 2, there was a statistical difference between different R. stricta organs that were examined in the present study. The R. stricta leaf extracts for the disinfection of date explants in tissue culture medium caused 40.33% more contamination than the root extract (Figure 2A).
Different solvents had different effects in obtaining R. stricta extract for the disinfection of tissue culture medium, as ethanol solvent appeared much more successful than aqueous solvent and could control tissue culture contaminants 58.5% more than aqueous solvent. In the present study, the contamination percentage was 70% in cultures containing aqueous extract and 44.16% in cultures containing ethanol solvent (Figure 2B).
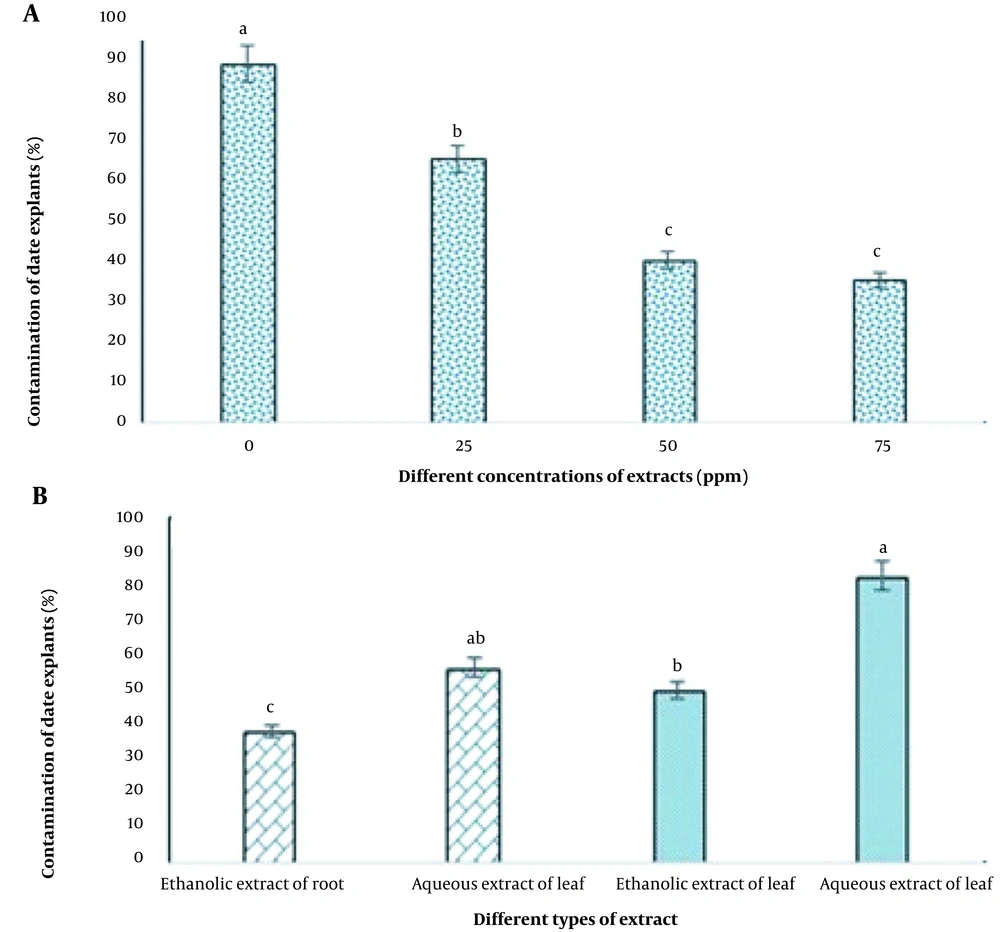
Figure 3A indicates that different concentrations of aqueous and ethanolic extracts significantly affected the contamination of date explants in tissue culture. The highest level of contamination (88.33%) was observed in the control treatment and the lowest (35%) at a concentration of 75 ppm of the extracts. However, there was no statistically significant difference between the two concentrations of 50 and 75 ppm (Figure 3A).
As shown in Figure 3B, the lowest level of contamination (38.33%) was obtained in the group disinfected with ethanolic root extract, while the aqueous leaf extract treatment resulted in the highest rate of contamination (88.33%) under tissue culture conditions.
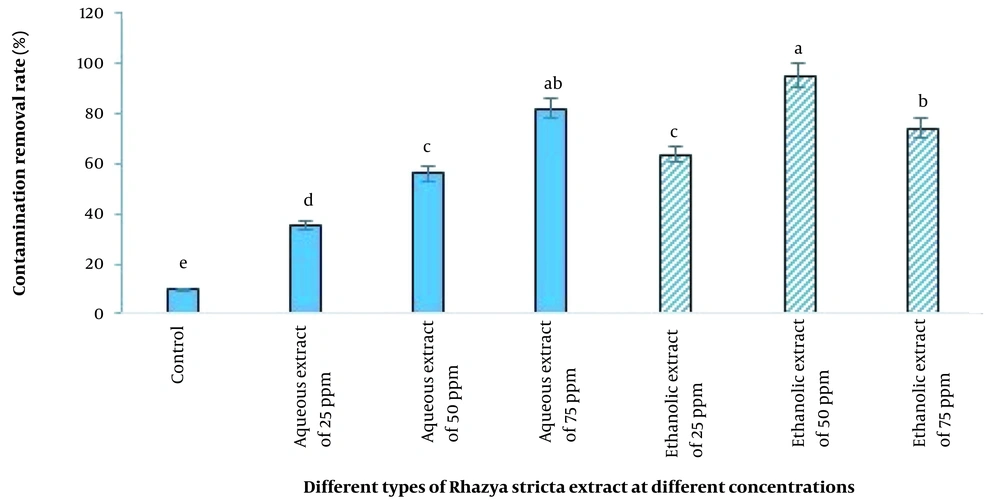
In the present study, the inhibitory effect of aqueous extract was less than that of ethanolic extract, and the ethanolic extract of R. stricta at a concentration of 50 ppm (95.33%) had the best effect on the date plant after adding the extract to the tissue culture medium, followed by the aqueous extract at a concentration of 75 ppm (80%). The lowest inhibition of fungal and bacterial growth was observed in the control treatment (9.66%) (Figure 4).
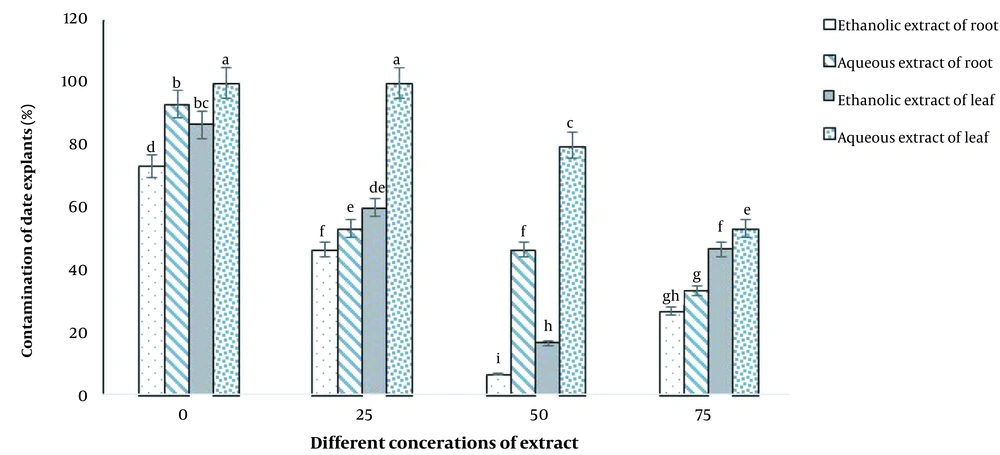
As shown in Figure 5, the highest level of contamination (100%) was obtained in the aqueous leaf extract treatment at concentrations of 0 and 25 ppm (Figure 6A), while the lowest level of contamination (6.66%) was observed in the culture containing ethanolic extract of R. stricta root at a concentration of 50 ppm (Figure 6B).
5. Discussion
It was found that bamboo plant explants taken from field and greenhouse conditions and added as 2 mL/L to the culture medium reduced the percentage of culture contamination from 58 to 52% and from 63 to 11%, respectively (39). Some research (40-43) was undertaken to apply different concentrations of turmeric powder, and carbendazim to the MS culture medium to remove contamination of the tissue culture medium. They showed that the application of turmeric powder and carbendazim in MS culture completely controlled the contamination of the culture medium. In the turmeric powder treatment, increasing concentration reduced the contamination percentage.
In many plants cultured in media containing antibiotics, especially gentamicin, the leaves became necrotic and fell off during growth, but they had more rooting than the treatment at the same concentration (44). Rhazya stricta leaf and fruit extracts demonstrated antibacterial activity against S. aureus, E. coli, P. aeruginosa, B. subtilis, S. pyogenes, and S. typhi (45). In this research, the leaf aqueous and ethanolic extracts of R. stricta were more effective than the root aqueous and ethanolic extracts against contamination by bacteria and fungi. Rhazya stricta leaf extract controlled bacterial growth on locally isolated meningococcal strains, increasing with concentration and treatment time (46). In this research, the contamination was controlled by increasing the concentration of ethanolic and aqueous extracts.
Chloroformic and methanolic extracts of R. stricta roots exhibited antimicrobial activity against B. subtilis, E. coli, S. aureus, and P. aeruginosa (11). The antibacterial activity of R. stricta leaf extracts at various concentrations was examined, showing that the organic alkaloid extract was most effective against E. coli (10). Rhazya stricta chloroformic and methanolic root fractions demonstrated antifungal activities against A. terreus, A. flavus, and C. albicans (47). Another study revealed that fractionated R. stricta methanolic and chloroformic samples had antifungal activity against T. longifusis, C. albicans, A. flavus, and F. solani (48). In this research, the ethanolic and aqueous root extracts had antimicrobial effects but less than the leaf extract.
Different solvents were used to extract the phytochemical content of R. stricta against S. typhimurium. The results showed that R. stricta, with the help of hydroalcoholic solvent, can be an effective plant in eliminating some bacteria, including S. typhimurium (9). This research also showed that the hydroalcoholic extract was more effective than the aqueous extract. Contrary to our results, a study (49) used two antibiotic mixtures to remove contaminants in a tomato culture medium. When both compounds were added to the culture medium, plant growth decreased, and contamination was not eliminated.
The contamination of the culture medium decreased with the addition of an antifungal agent, and the number of leaves was less than in control without fungus and more than in control with fungus (50). The presence of antibacterial agents in the culture medium reduced the contamination and was suitable for plant growth. However, the presence of antibacterial agents disrupts the growth system and causes stress in plant growth, including the number of leaves.
5.1. Conclusions
When the purpose of tissue culture is the commercial propagation of plants, contamination control and preparation of sterile explants are greatly important. Disinfectants are essential for controlling bacterial and fungal contamination in the in vitro culture. Based on the research results, the best result in controlling contamination in palm tissue culture was obtained from ethanolic root extract, and the worst was obtained from the aqueous leaf extract. In general, the ethanolic extract was more potent than the aqueous extract, and the root extract was more potent than the leaf extract.