1. Background
Breast cancer is a major malignancy in women and the third cause of cancer death worldwide. In recent years, incidence of breast cancer has gradually decreased in developed countries, but this cancer is still a common malignancy in Iran (1). Hereby, the exact etiology of breast cancer remains unclear; however, environmental and genetic factors are important in tumorigenesis of breast cancer. Due to the lack of symptoms in the early stages of breast cancer, this malignancy is usually diagnosed in the advanced stages (2). Chemotherapy, radiotherapy, and surgery are current common treatment approaches for breast cancer (3). However, reduced quality of life and numerous side effects have decreased patients’ desire to receive these treatments. Moreover, increased drug resistance to current chemotherapeutic compounds has led to extensive efforts to identify and introduce natural anti-cancer compounds (4, 5).
From past centuries, herbal medicines and other natural compounds have been widely used to prevent and treatment of numerous human diseases. Therefore, in recent years, numerous studies have identified properties and effects of herbal medicines (6, 7). In addition, due to high economic efficiency, as well as lower side effects, there is a great deal of interest in the use of herbal medicines and other natural compounds (8, 9).
Olive is one of the important herbal medicines used broadly due to its anti-inflammatory, antioxidant, and anti-cancer activity. Hydroxytyrosol is an important natural compound in olive oil, which indicates high anti-cancer activity through regulation of numerous cellular pathways, such as cell cycle arrest and apoptosis induction (10, 11). So far, many studies have reported anti-cancer activity of hydroxytyrosol against numerous human malignancies (12, 13). In a recent study, León-González et al. demonstrated that cytotoxic effect of hydroxytyrosol on prostate cancer cells was associated with regulation of key phosphorylation in ERK1/2 and AKT signaling pathways (14). In another study, Oktay and Tuğrul reported that the cytotoxic effect of hydroxytyrosol could occur through induction of apoptosis and modification of ERK1/2 signaling pathways (15). Han et al. suggested that hydroxytyrosol exhibited significant arrest of G1 to S phase transition and increase of cell number in G0/G1 phase in breast cancer cells (16). This evidence suggests that hydroxytyrosol can be considered an important anti-cancer agent with low side effects. However, the exact anti-cancer activity of hydroxytyrosol and underlying molecular mechanisms remain unknown.
2. Objectives
Due to high prevalence and large number of patients with breast cancer in the world, investigation of various function mechanisms of hydroxytyrosol is important for development of therapeutic approaches to breast cancer. However, toxicity and anti-proliferative potential of hydroxytyrosol against breast cancer cells and involved molecular mechanisms have not been fully elucidated. Therefore, we aimed to investigate in vitro effects of hydroxytyrosol on the viability, proliferation, and apoptosis via the expression of apoptotic genes in breast cancer cell lines.
3. Methods
3.1. Cell Culture
Two breast cancer cells, MDA-MB-231 and MCF-7, were obtained from Iranian Pasteur Institute, Iran, Tehran. The breast cancer cell was cultured in T25 cell culture flask containing a complete RPMI-1640 medium supplemented by 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cancer cells were grown in standard conditions (37°C with 5% CO2). The culture medium was substituted every two days, and the cancer cells were passaged at ~80 - 90% confluency. The cultured cancer cells in the logarithmic growth phase were used for further investigations (17).
3.2. Cell Viability Assay
The breast cancer cells were seeded in a 96‐well plate (1.5 × 104 cells/well) and incubated in standard conditions. The cancer cells were treated with hydroxytyrosol, and again incubated for 24, 48, and 72 hours in standard conditions. After this time, a fresh FBS-free RPMI-1640 medium (200 μL) containing MTT solution (50 μL) was substituted with old medium, and again incubated for four hours in standard conditions. The old medium was substituted with dimethyl sulfoxide (DMSO), and incubated for 30 minutes in standard dark conditions. Finally, the viability of the cancer cells was calculated by optical density (OD) of all wells at 570 nm wavelength.
3.3. Apoptosis Quantification
The breast cancer cells were seeded in a 6‐well plate (1.5 × 105 cells/well) and incubated for 24 hours in standard conditions. The cancer cells were treated with hydroxytyrosol and again incubated for 72 hours in standard conditions. After this time, the cancer cells were resuspended by a binding buffer (400 μL). Also, fluorescein isothiocyanate (FITC) conjugated Annexin V (5 μL), and propidium iodide (10 μL) were added to the cellular suspension, and incubated for five minutes in standard dark conditions. Finally, the flow cytometry instrument was applied to the measurement of apoptosis rate in breast cancer cells.
3.4. Gene Expression Analysis
The breast cancer cells were seeded in a 6‐well plate (1.5 × 105 cells/well) and incubated for 24 hours in standard conditions. The cancer cells were treated with hydroxytyrosol, and again incubated for 72 hours in standard conditions. Total RNA extraction was conducted by TRIzol reagent, and cDNA synthesis was performed by reverse transcriptase enzyme. Quantitative real-time PCR (qPCR) was applied to investigate mRNA expression of BAX, BCL2, and CASP3 genes by specific primers (Table 1). The amplification process was performed in 10 μL total volume (1 μL cDNA, 0.5 μL forward primer, 0.5 μL reverse primer, five μL PCR pre‐Mix, and three μL distilled sterile water) by following one cycle for initial denaturation (94°C for 1 minute), and 40 cycles for denaturation (94°C for 10 seconds), annealing (30 seconds), and extension (72°C for 20 seconds). Moreover, mRNA expression of ACTB gene was considered endogenous control.
| Gene and Primer Sequence | Ta | Tm | PS (bp) | References |
|---|---|---|---|---|
| BAX | 59°C | 155 | (18) | |
| F: 5’-CCCGAGAGGTCTTTTTCCGAG-3’ | 60°C | |||
| R: 5’-CCAGCCCATGATGGTTCTGAT-3’ | 60°C | |||
| BCL2 | 54°C | 223 | (19) | |
| F: 5’-GATGGGATCGTTGCCTTATG-3’ | 56°C | |||
| R: 5’-GCGGAACACTTGATTCTGG-3’ | 56°C | |||
| CASP3 | 57°C | 122 | (20) | |
| F: 5’-GCAGGCTCTGGATCTCGGC-3’ | 59°C | |||
| R: 5’-GCTGCTTGCCTGTTAGTTCGC-3’ | 59°C | |||
| ACTB | 58°C | 186 | (21) | |
| F: 5’-AGAGCTACGAGCTGCCTGAC-3’ | 61°C | |||
| R: 5’-AGCACTGTGTTGGCGTACAG-3’ | 59°C |
Sequence of the Used Primers for Quantification of Apoptotic Genes Expression
3.5. Statistical Analysis
The GraphPad Prism software version 6 was applied for statistical analysis. The obtained data from three times repeated experiments were statistically analyzed by one‐way analysis of variance (ANOVA), Student’s t-test, and Tukey (post hoc). The statistical significance was considered P < 0.05.
4. Results
4.1. Cancer Cells’ Viability
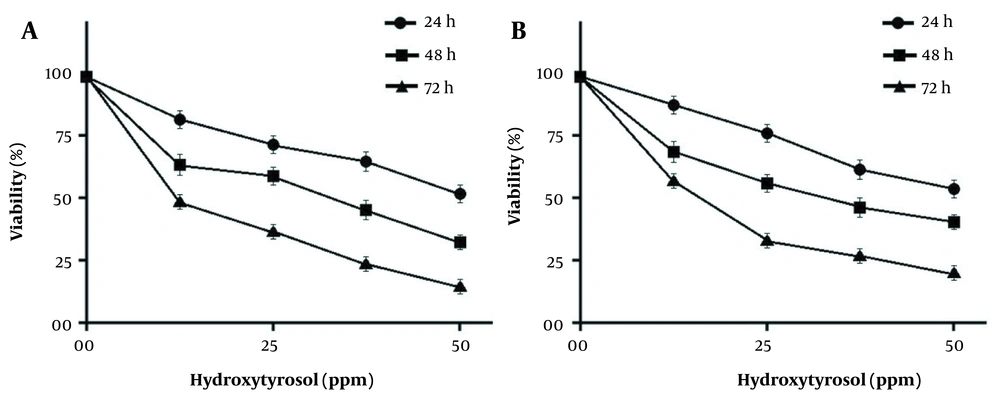
Hydroxytyrosol demonstrated anti-proliferative effects against both MDA-MB-231 and MCF-7 breast cancer cells in a time- and dose-dependent manner. The cancer cells’ viability significantly decreased after treatment with high concentrations of hydroxytyrosol for a longer time. The half-maximal inhibitory concentration (IC50) of hydroxytyrosol on MDA-MB-231 and MCF-7 cancer cells after 72 hours were 12 ppm and 14 ppm, respectively (Figure 1).
4.2. Apoptosis in Cancer Cells
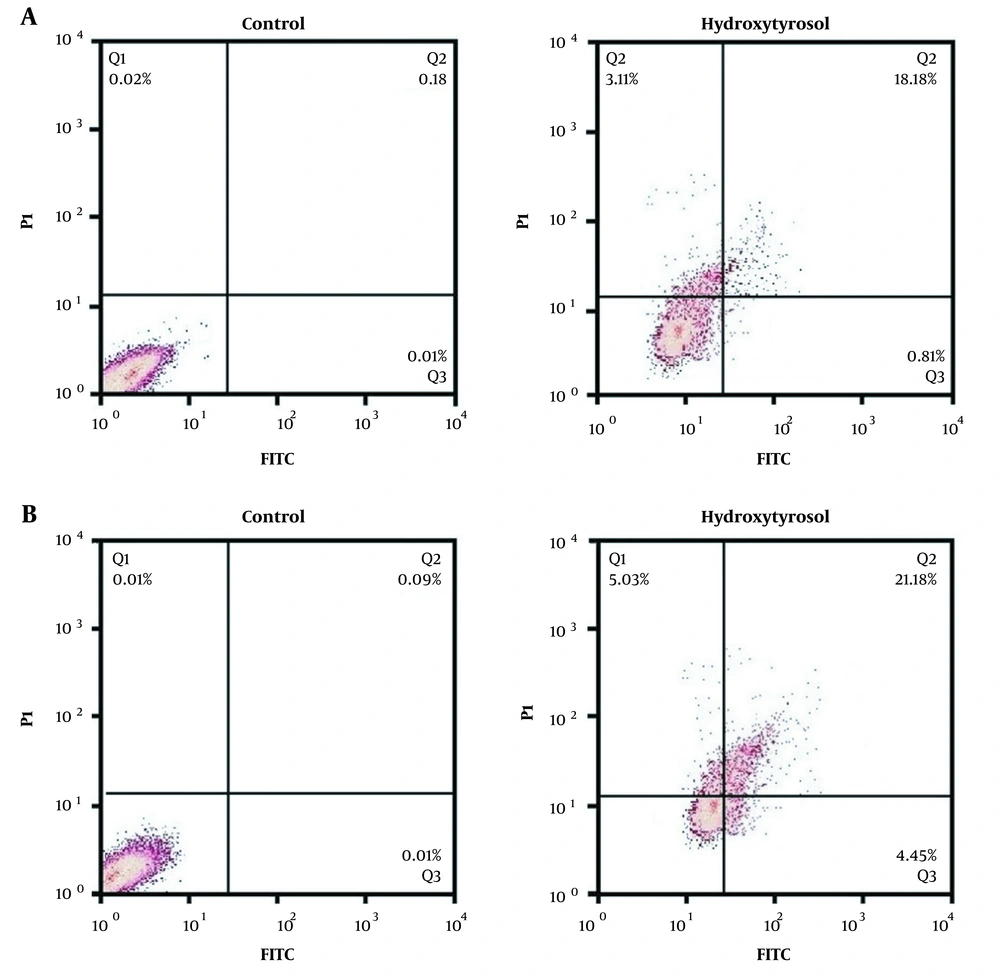
Apoptosis percentage (early and late) increased significantly in treated MDA-MB-231 (12 ppm) and MCF-7 (14 ppm) cancer cells as compared to the untreated cells. The percentage of total apoptosis in the untreated MDA-MB-231 cancer cells was 0.23%, whereas this proportion increased to 22.1% in the treated cancer cells with hydroxytyrosol. In addition, the percentage of total apoptosis in the untreated MCF-7 cancer cells was 0.11%, whereas this proportion increased to 30.66% in the treated cancer cells with hydroxytyrosol (Figure 2).
4.3. Expression of Apoptotic Genes
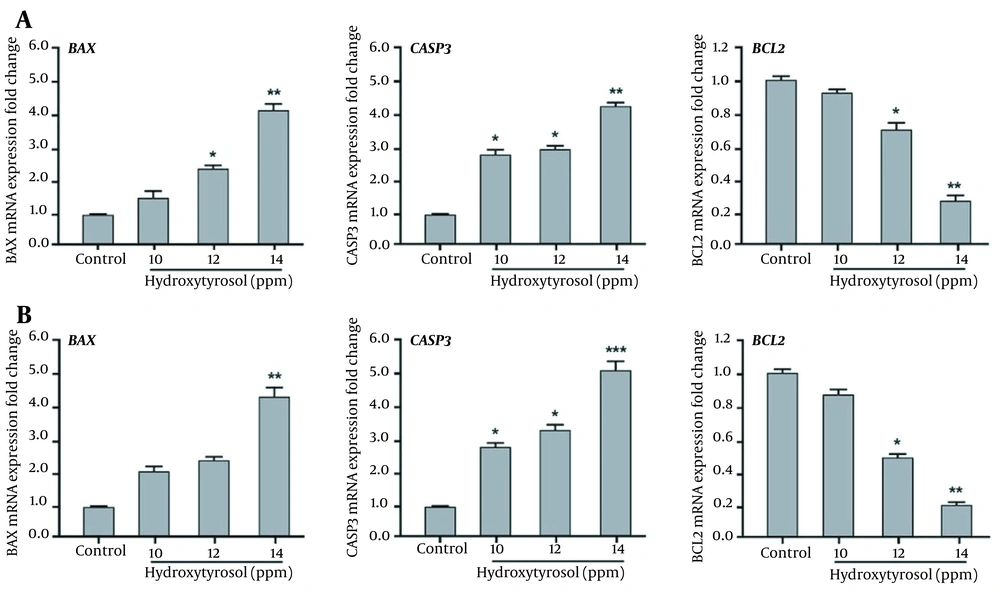
The expression of apoptotic genes significantly changed after treatment with hydroxytyrosol. Hydroxytyrosol significantly upregulated mRNA expression of BAX and CASP3 pro-apoptotic genes in both MDA-MB-231 (12 ppm) and MCF-7 (14 ppm) breast cancer cells. In contrast, mRNA expression of BCL2 anti-apoptotic gene was significantly downregulated after treatment of the breast cancer cells with hydroxytyrosol (Figure 3).
5. Discussion
Resistance to the common chemotherapeutic drugs is a major obstacle to managing and treating human malignancies. Cancer cells acquire the ability to resist chemotherapeutic drugs through numerous mechanisms such as hypoxia, drug inactivation, microRNAs, drug efflux, drug-target alteration, extracellular matrix alterations, growth factors, and cytokines production and dysregulation pathways of cell death and survival (22, 23).
In recent years, many efforts have been made to overcome drug resistance in cancer cells by targeting various mechanisms of resistance. Moreover, many studies have been performed to introduce novel natural compounds and derivatives as potential chemotherapeutic agents. In particular, pharmacological studies have significantly focused on various herbal medicine and bioactive compounds over the past two decades (24, 25). This special attention to the natural compounds and herbal medicine is due to their low side effects, high efficiency, low costs, as well as targeting of cancer-related signaling pathways (26, 27).
Hydroxytyrosol is one of the most notable natural compounds which has been introduced in recent years due to its beneficial properties. Olive oil contains large amounts of hydroxytyrosol that indicate appropriate anti-tumor and antioxidant activity. Previous studies have suggested that hydroxytyrosol decreases cancer cells’ growth and proliferation through cell cycle arrest and apoptosis induction (28, 29). In the present study, we evaluated anti-proliferative effects of hydroxytyrosol against two MDA-MB-231 and MCF-7 breast cancer cell lines and induction of apoptosis and expression of pro-apoptotic genes. Our results indicated a high anti-proliferation activity by hydroxytyrosol against both breast cancer cell lines.
In recent years, numerous studies have been reported on anti-tumor and anti-proliferation activity of hydroxytyrosol. Furthermore, identification of involved mechanisms in hydroxytyrosol function against cancer cells is the main aim of recent studies (30, 31). In a study, Jadid et al. reported that hydroxytyrosol increased the percentage of apoptosis in a pancreas cancer cell line (32). They also suggested that induction of apoptosis by this compound could be due to upregulation of pro-apoptotic genes as well as downregulation of anti-apoptotic genes (32). In a similar study, Ahmadi et al. reported that hydroxytyrosol presented an anti-proliferation activity against colorectal cancer cells through apoptosis induction and cell cycle arrest (33). In another recent study, Hormozi et al. demonstrated that hydroxytyrosol significantly increased mRNA expression of apoptotic genes (CASP3, BAX, BCL2) in a human colorectal cancer cell line (34). Our study confirmed the results of previous studies. This evidence suggests that hydroxytyrosol, as a natural compound, can be helpful in cancer treatment.
5.1. Conclusions
In general, we suggest that hydroxytyrosol potentially has an acceptable anti-proliferation activity on breast cancer cells. Furthermore, hydroxytyrosol may induce apoptosis in breast cancer cells through expression modifications of apoptotic genes. However, more studies are required to identify mechanisms of hydroxytyrosol anti-proliferation activity, which may be used as an anti-cancer therapeutic compound after clinical studies.