1. Background
The world population is proliferating, and it is predicted to reach 9.5 billion individuals by 2050. On the other hand, food production diminishes due to various abiotic stresses (1). Therefore, the facilitation of the yield loss in crops is among the global concerns for the countries; therefore, they can supply human food requirements (2). Crops are mainly affected by stresses, such as cold temperature (3), salinity (4), drought (5), flooding (6), oxidative stress (7), pathogens (8), and heavy metal toxicity (9). Human activities have exacerbated the present stress-inducing factors. All these stresses are menaces to plants and prevent them from achieving their maximum genetic potential. They have also limited crop production all over the world.
Citrus is among the three significant fruits in the world (10). Citrus cultivation, especially orange, is a major industry and an essential part of the United States, Brazil, Mexico, China, India, Iran, and numerous Mediterranean countries, such as Spain and Greece (11). Citrus is cultivated in more than 100 countries, mainly situated in tropical and subtropical regions with suitable soil and climate (12). Citrus is valuable both in terms of fresh consumption and in the processing industry. The citrus processing industry has focused on juice and essence production for years. It is estimated that 33% of citrus harvested globally is used for juice production (11). Iran is ranked 5th regarding citrus production worldwide, and climatic diversity and production of the best citrus varieties have given the country a good advantage. Citrus is produced in seven provinces of Iran. Mazandaran province is regarded as the main center of citrus production with 1.5 - 1.7 million tons annually, followed by Golestan, Guilan, Hormozgan, and Kerman provinces.
Weather fluctuation is one of the significant obstacles to the production of crops, especially citrus (13). Low temperature is a challenging factor for commercial citrus production, as citrus species are sensitive to low temperatures and harvest in winter (14). Various citrus species are susceptible to -2.2°C and lower temperatures. However, some commercial citrus species are cultivated in regions with an increased risk of freezing (15). The high risk of freezing in winter during citrus growth is a big issue in the Caspian Seabank, along the coasts of the Black Sea, coastal regions of France, the Adriatic Sea in Yugoslavia, some parts of Turkey, Spain, Israel, and, Greece. A sudden decline in the below-freezing temperature threshold renders the trees susceptible to freezing. Although citrus growers mitigate the freezing risk using various methods, such as sprinkler irrigation, protective materials, and costly measures, the development of cold-tolerant citrus cultivars using genetic engineering methods provides long-term protection against cold (16, 17).
Advances in biotechnology and identification of the genes regulating tolerance to different environmental conditions, such as cold temperature, using genetic engineering and their introduction into the commercial citrus cultivars might be a solution for this issue (18). Noting the periodical occurrence of chilling stress in the north of Iran (2007, 2013, 2016, and 2018) and the resulting economic and social challenges, efforts to alleviate this damage seem necessary. Due to the generic nature of cold stress tolerance (polygenic) in citrus species, it appears that cold patience and knowledge about the mechanisms involved in it to select suitable cultivars are among the significant priorities for citrus breeding programs in subtropical regions. The use of suitable cultivars is a reasonable and cheap way to reach sustainable production in an area, and the selection of tolerant species and implementation of good farming practices might also be effective.
There are various methods for the analysis of traits, and the researcher chooses one of them according to their aims. Simple correlation, regression models, and pathway analysis are commonly used to analyze physiological traits (19). The investigation of stress tolerance relationships and their interactions is critically important to obtain high levels of tolerance (20). In general, the correlation coefficient is mainly used to describe the relationship among traits. However, this coefficient might be misleading in some instances, as a high correlation between two traits might be due to the indirect effects of traits on each other (21). In these types of studies, selection based on simple correlation alone cannot provide optimum results. Therefore, it is essential to determine the direct and indirect effects of some traits affecting other important traits. In this regard, stepwise regression and pathway analysis are of utmost importance, and various studies have been performed to investigate the causality relationships of traits (22). In stepwise regression, all independent variables enter the model, and those independent variables with no significant effect on the dependent variable will be removed from the model. This method is a combination of forward and backward regressions. In forward regression, no variable is present in the model at the beginning, and the first variable entering the model is the one with the highest correlation with the dependent variable (23).
Stress tolerance or the median lethal dose (LT50) is a quantitative trait controlled by multiple genes. This trait is highly influenced by the environment. Various characteristics affect the LT50 alone or in combination with each other (24). For the purpose of the determination of the role of physiological traits in improved stress tolerance and increased selection efficiency by a limited number of traits, which are important parameters to achieve breeding goals, stepwise selection of variables in a multiple linear regression might be used (25). The investigation of the traits affecting stress tolerance in citrus using stepwise regression analysis is not available in the literature.
2. Objectives
The present study was conducted to identify the physiological and biochemical traits of some citrus cultivars with the greatest influence on stress tolerance and determine the direct and indirect effects of these traits on the LT50 using integrated pathway analysis (i.e., stepwise regression and causality coefficient). The findings of the present study might be used to develop breeding programs to improve citrus stress tolerance.
3. Methods
3.1. Experiment Conditions and Treatments
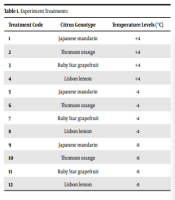
The present study was conducted at the Horticulture Laboratory of Guilan University in 2021 to investigate the biochemical traits of four citrus species (i.e., Japanese mandarin, Thomson orange, Ruby Star grapefruit, and Lisbon lemon) at three temperature levels (i.e., -8, -4, and +4°C) (Table 1). Two-year-old seedlings were selected and placed in 10 kg pots filled with the typical soil of the region (i.e., silty loam texture) (26). Before the application of treatments and for the adaptation of the plant material to cold, the seedlings were transferred to temperature conditions (27) and then to a storage room with a +4°C temperature and 65 ± 5% relative humidity. This relative humidity was chosen according to the moisture required for citrus growth in the North of Iran. The temperature of the test chamber was +4°C, which decreased by 1.5°C every hour. The samples were kept at the aforementioned temperature treatments for 10 hours when the test chamber device reached that temperature, and the traits were measured after this period (i.e., 10 hours) (28).
| Treatment Code | Citrus Genotype | Temperature Levels (°C) |
|---|---|---|
| 1 | Japanese mandarin | +4 |
| 2 | Thomson orange | +4 |
| 3 | Ruby Star grapefruit | +4 |
| 4 | Lisbon lemon | +4 |
| 5 | Japanese mandarin | -4 |
| 6 | Thomson orange | -4 |
| 7 | Ruby Star grapefruit | -4 |
| 8 | Lisbon lemon | -4 |
| 9 | Japanese mandarin | -8 |
| 10 | Thomson orange | -8 |
| 11 | Ruby Star grapefruit | -8 |
| 12 | Lisbon lemon | -8 |
Experiment Treatments
3.2. Measurement of Traits
Ion leakage, relative water content, and LT50 traits were immediately measured after the application of each temperature treatment. For the measurement of the traits, such as leaf chlorophyll and carotenoid, total flavonoid, peroxidation of membrane lipids, and the antioxidant activity of catalase, superoxide dismutase, and ascorbate peroxidase after the induction of stress, the samples were immediately frozen in liquid nitrogen and kept at -80°C to be measured later. The biochemical traits were measured according to Table 2.
| Studied Trait | Measurement Method |
|---|---|
| Total flavonoid | Campos et al. (29) |
| Malondialdehyde | Campos et al. (29) |
| Ascorbate peroxidase | Maehly and Chance (30) |
| Catalase | Clairborne (31) |
| Chlorophyll a | Barnes et al. (32) |
| Chlorophyll b | Barnes et al. (32) |
| Total chlorophyll | Barnes et al. (32) |
| Carotenoid | Barnes et al. (32) |
| Superoxide dismutase | Wu et al. (33) |
| RWC | Ritchie et al. (34) |
| Ion leakage | Sullivan and Ross (35) |
| LT50 | Lim et al. (36) |
| Anthocyanin | Wagner (37) |
| Proline | Bates et al. (38) |
| Glycine-betaine | Grieve and Grattan (39) |
| Hydrogen peroxide | Alexieva et al. (40) |
Measurement of the Studied Traits
3.3. Statistical Analysis
Prior to performing the analysis of variance on the data, the Roc Univariate command was employed to check the normality of data distribution, and ensuring this issue was followed by the analysis of descriptive statistics and correlation coefficient. On the other hand, stepwise regression analysis was employed at a significance level of 1% to specify the key traits affecting stress tolerance in these cultivars. The causal analysis method (based on the traits with the most justification in stress tolerance) was used to specify the direct and indirect effects of each of the traits included in the stress tolerance model. All the aforementioned calculations (i.e., checking the normality of data distribution, determining simple correlation coefficients, stepwise regression analysis, and causal analysis) were conducted using SPSS software (version 24).
4. Results
Table 3 shows descriptive statistics related to the studied traits. These results indicated a suitable variation among the studied populations. According to the standard deviation value, relative moisture, superoxide dismutase, and ascorbate peroxidase traits had the highest variations. Relative moisture content had a range of variation equal to 179.5. This value was reported as 109 and 55 for superoxide dismutase and ascorbate peroxidase, respectively, indicating a high variation. In this study, the effect of citrus cultivar and temperature on the biochemical traits was studied for the first time. The results showed that the traits of various citrus cultivars had a wide range of biochemical properties. Descriptive statistics show general information on the studied traits and help the researchers to have a better and more precise knowledge of the studied traits.
| Trait | Mean ± Standard Variation | Range | Minimum | Maximum |
|---|---|---|---|---|
| Total flavonoid | 11.92 ± 9.55 | 30.36 | 3.06 | 33.42 |
| Ascorbate peroxidase | 31.55 ± 15.75 | 55 | 12 | 67 |
| Catalase | 30.42 ± 3.01 | 10.5 | 26.6 | 37.1 |
| Malondialdehyde | 0.66 ± 0.35 | 1.4 | 0.22 | 1.62 |
| Chlorophyll a | 3.18 ± 3.11 | 10.45 | 0.18 | 10.63 |
| Chlorophyll b | 0.83 ± 0.7 | 2.9 | 0.16 | 3.06 |
| Total chlorophyll | 4.02 ± 3.52 | 12.5 | 0.73 | 13.22 |
| Carotenoid | 1.56 ± 1.11 | 3.52 | 0.34 | 3.86 |
| Superoxide dismutase | 126.4 ± 29.84 | 109 | 53 | 162 |
| RWC | 93.12 ± 56.64 | 179.5 | 28 | 207.5 |
| Ion leakage | 26.7 ± 17.1 | 54.93 | 13.89 | 68.82 |
| LT50 | 5.54 ± 3.13 | 10.73 | 12 | 1.27 |
| Anthocyanin | 0.164 ± 0.07 | 0.202 | 0.035 | 0.238 |
| Proline | 13.2 ± 3.57 | 15.3 | 7 | 22.3 |
| Glycine-betaine | 6.22 ± 4.11 | 12.94 | 1.91 | 14.85 |
| Hydrogen peroxide | 0.24 ± 0.14 | 0.47 | 0.035 | 0.51 |
Descriptive Statistics Related to Various Traits in Citrus Cultivars
4.1. Correlation Coefficients
The correlation coefficient was used to determine the extent of the relationship between the linear changes in the two traits. This coefficient described the degree of linear relationship and direction of changes between two traits. Table 4 shows Pearson’s correlation coefficients among the studied traits. The results showed that the LT50 with total flavonoid (0.443**), chlorophyll a with chlorophyll b (0.613**), carotenoid with chlorophyll a (0.929**), chlorophyll b (0.573**), and total chlorophyll (0.849**), relative moisture content with malondialdehyde (0.559**), glycine-betaine with catalase (0.919**), hydrogen peroxide with total flavonoid (0.405**), and catalase (0.611**) had the highest positive and significant correlations, which indicated that improvement in each of these traits might lead to enhanced stress tolerance.
| Traits | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1) Total flavonoids | 1 | |||||||||||||||
| 2) AXP | 0.211ns | 1 | ||||||||||||||
| 3) CAT | 0.081ns | 0.765 ** | 1 | |||||||||||||
| 4) Malondialdehyde | 0.027 ns | 0.108 ns | 0.236 ns | 1 | ||||||||||||
| 5) Chlorophyll a | 0.270 ns | 0.262 ns | 0.106 ns | 0.457 ** | 1 | |||||||||||
| 6) Chlorophyll b | 0.142 ns | 0.136 ns | 0.056 ns | 0.141 ns | 0.613 ** | 1 | ||||||||||
| 7) Total chlorophyll | 0.303 ns | 0.316 ns | 0.138 ns | 0.356 * | 0.835 ** | 0.543 ** | 1 | |||||||||
| 8) Carotenoid | 0.202 ns | 0.464 ** | 0.114 * | 0.464 ** | 0.929 ** | 0.573 ** | 0.849 ** | 1 | ||||||||
| 9) SOD | 0.134 ns | 0.179 ns | 0.343 * | 0.050 ns | 0.380 * | 0.184 ns | 0.429 ** | 0.386 * | 1 | |||||||
| 10) RWC | 0.255 ns | 0.185 ns | 0.040 ns | 0.559 ** | 0.114 ns | 0.094 ns | 0.001 ns | 0.103 ns | 0.061 ns | 1 | ||||||
| 11) Ion leakage | 0.310 ns | -0.328 ns | 0.481 ** | 0.048 ns | 0.354 * | 0.237 ns | 0.178 ns | 0.246 ns | 0.289 ns | 0.639 ** | 1 | |||||
| 12) LT50 | 0.443 ** | -0.165 ns | 0.237 ns | 0.332 * | 0.101 ns | 0.162 ns | 0.187 ns | 0.095 ns | 0.264 ns | 0.204 ns | 0.115 ns | 1 | ||||
| 13) Anthocyanin | 0.201 a | 0.572 ** | 0.784 ** | 0.056 ns | 0.096 ns | 0.120 ns | 0.118 ns | 0.092 ns | 0.093 ns | 0.042 ns | 0.418 * | 0.308 ns | 1 | |||
| 14) Proline | 0.013 ns | 0.157 ns | 0.464 ** | 0.495 ** | 0.113 ns | 0.217 ns | 0.227 ns | 0.087 ns | 0.252 ns | 0.138 ns | 0.227 ns | 0.264 ns | 0.347 * | 1 | ||
| 15) Glycine-betaine | 0.284 a | 0.646 ** | 0.919 ** | 0.239 ns | 0.130 ns | 0.109 ns | 0.167 ns | 0.059 ns | 0.304 ns | 0.13 ns | 0.411 * | 0.402 * | 0.804 ** | 0.464 ** | 1 | |
| 16) Hydrogen peroxide | 0.405 * | 0.161 ns | 0.611 ** | 0.133 ns | 0.562 ** | 0.121 ns | 0.526 ** | 0.376 * | 0.341 * | 0.181 ns | 0.429 * | 0.154 ns | 0.520 ** | 0.156 ns | 0.561 ** | 1 |
Pearson’s Correlation Coefficients of the Studied Traits in Citrus Cultivars a
4.2. Stepwise Regression
Stepwise regression was first performed to determine the most important traits influencing the LT50, total flavonoid, and proline and the elimination of negligible variables and pathway analysis. Table 5 shows the results of stepwise regression with the LT50 as the independent variable and other traits as the dependent variables. The LT50, total flavonoid, and proline with partial regression coefficients of 88.5, 96%, and 93.8% entered the model, respectively. In general, the first and second variables that entered the model were total flavonoid and proline, which had the highest share in the description of the LT50. In addition, due to the high correlation coefficient of these traits with the LT50, they are the most important traits in this study and should be noted in citrus breeding programs.
Results of Stepwise Regression for Median Lethal Dose, Total Flavonoid, and Proline as Dependent Variables and Other Traits as Independent Variables
4.3. Path Analysis
The variables that entered the model were subjected to pathway analysis to further interpret the results. Table 6 shows that all the coefficients of the direct path (except for proline) are significant in the final pattern. Overall, these traits described 77.5% of changes in the LT50. Table 6 shows the direct and indirect effects of the traits on the LT50, according to which total flavonoid and proline had the highest positive and significant effect on the other biochemical traits.
| Variable | Direct Effect | Indirect Effect | |||
|---|---|---|---|---|---|
| Total Flavonoid | Proline | LT50 | Residual Effects | ||
| Total flavonoid | 0.608 ** | - | -0.374 ns | 1.465 ** | 1.091 ** |
| Proline | 0.559 ** | -0.061 ns | - | 0.079 ns | 0.018 ns |
| LT50 | 0.414 ** | 0.146 ** | 0.236 ns | - | 0.382 ** |
Direct and Indirect Effects of Median Lethal Dose, Total Flavonoid, and Proline a
5. Discussion
Cold stress can be classified as chilling (0 - 15°C) and freezing (< 0°C) stresses. Generally, plants originating from temperate regions, such as spinach and arabidopsis, exhibit a variable degree of chilling tolerance and can increase their freezing tolerance during exposure to chilling and nonfreezing temperatures. This process is known as cold acclimation (41). On the other hand, plants of subtropical origins are sensitive to chilling stress and lack the cold acclimation mechanism (42). Information regarding the physiological traits of such stepwise regression will provide the knowledge to develop cold-tolerant cultivars.
Knowledge of various physiological aspects helps researchers to select breeding strategies for citrus. The present study was applied research to facilitate the selection of the best citrus genotypes. Overall, the results of Pearson’s correlation, stepwise regression, and pathway analysis confirmed each other.
Pearson’s correlation coefficients described the degree of linear relationship and direction of changes between two traits. Quantification is very important in breeding (25). Table 4 shows Pearson’s correlation coefficients of the studied traits. The results showed that the LT50 with total flavonoid, chlorophyll a with chlorophyll b, carotenoid with chlorophyll a, chlorophyll b, and total chlorophyll, relative moisture content with malondialdehyde, glycine-betaine with catalase, hydrogen peroxide with total flavonoid, and catalase had the highest positive and significant correlations, which indicated that improvement in each of these traits might lead to enhanced stress tolerance. Abouzari et al. (43) showed a positive and significant correlation between ion leakage and leaf water core in citrus cultivars. A positive correlation between proline and carbohydrates was reported in Page mandarin by Tadjvar et al. (26). Table 4 shows a significant positive correlation between malondialdehyde and proline content (0.99; P < 0.01); the aforementioned results are consistent with the results of a study performed by Li et al. (41).
Correlation between the traits might help breeders in indirect selection for important stress traits through other traits that are easier to measure (44). Although the correlation coefficients of physiological traits help determine the traits related to stress, they fail to describe the relationship correctly, and it is required to determine the direct and indirect effects of these traits (44, 45). Therefore, stepwise regression is used to select valuable variables among numerous measure traits to identify traits with the highest share in the description of stress tolerance (46). This method can eliminate traits that are ineffective or have a negligible effect on tolerance in the regression model and only identify traits that significantly describe the changes (47). In this study, due to the numerousness of traits with significant and positive correlation with the LT50, it is reasonable to further investigate the traits using other statistical methods to determine the important traits affecting citrus performance. Therefore, integrated pathway analysis was used for further evaluation to obtain more sound information regarding the traits and their effect on citrus LT50.
5.1. Conclusions
Based on the results of this study, it was concluded that there is a clear link between the expression of the LT50 and other physiological traits examined under cold stress at -8°C. Total flavonoid and proline show a positive correlation with the LT50 under cold stress in citrus. The aforementioned results provide necessary information for understanding the mechanism of the stress response and the adaption of citrus to cold conditions. This study will also help breeders and molecular biologists to evaluate existing germplasms for cold tolerance and/or develop new stress-resistant cultivars.