1. Background
Cancer is considered as the leading cause of death in the sense that there have been almost 13 million newly diagnosed cases and 8 million deaths within a year (1). One of the most widely used breast cancer cell line models is the breast adenocarcinoma cell line (MCF-7), which is used for looking at anti-cancer drugs (2). Women in the United States suffer from MCF-7 as one of the most prevalent women's cancers, with 266,120 new cases and 40,920 breast cancer deaths in 2017 (3). According to Jemal et al.'s final report, the yearly rate of breast cancer is enhancingby 0.4% globally (4). Chemotherapy is often used as adjuvant anti-cancer therapy. Chemotherapy is applied to most patients prior to or after surgery (1).
Attempts to explore new cancer chemotherapeutics have led to the introduction of natural products. The substances obtained from plants have been determined as models for developing new drugs. The adaptation of plants results in synthesizing second metabolites whose compounds additionally exist in pharmacological activities, amongst which the antibacterial, antihypertensive, anti-inflammatory, and anti-cancer tasks are the most regularly reported ones (5).
Taxol (generic name paclitaxel) is a clinically effective antitumor drug being approved for treating breast carcinomas (6). Also, taxol is a poly-oxygenated cyclic di-terpenoid with a characteristic taxane ring system. This drug has been the most efficient and pervasively utilized chemotherapeutic for treating cancers and virus-related sarcomas. Besides, Kumar et al.'s therapy of cells with taxol hinders the regular reconstruction of the microtubule network and also prevents the regular spindle development at the metaphase required for mitosis as well as cell expansion (7). This yields the cells' arrestment at the G2/M phase of the cell cycle and apoptotic cell death as a consequence (8). Taxol and other hydrophobic antitumor drugs are substrates for P-glycoprotein, which is the MDR1 gene product. Along with the overexpression of P-glycoprotein, other actively involved mechanisms consist of c-erbB2/neuoverexpression in breast cancer cells, changes in L-tubulin isotypes, anomalies in tubulin and also particular changes in many properties of signal transduction paths. The activation of Raf-1 and mitogen-activated protein kinase (MAP kinase), which are molecular changes in molecules engaged with the cascades of signal transduction, has resulted after treating cells that are sensitive to drugs with taxol (9).
Vinblastine and Vincristine effectively applied in chemotherapy regimens, are recognized as the first two vinca alkaloid compounds. These agents resolve to apprehend the separating cells at metaphase by binding to the β-subunit of tubulin heterodimers to avoid polymerization and incorporation into microtubules (10). The different vinca alkaloids have their unique properties. For example, vinblastine has an angiogenesis inhibitory effect. It is also related to the anti-diuretic hormonal agent secretion and angina, which are used to deal with Hodgkin's illness, non-Hodgkin's lymphoma, and breast cancer (11). Vincristine contains a high fondness for tubulin dimers, and its response to dimers is rapidly reversible. This implies that a vincristine particle will affix to a dimer at one site, break off, and afterward reattach to an additional site, resulting in two sites per dimer "poisoned" and incapable of reassembling into the protein. Hence, vincristine is potentially able to destabilize tubulin (12). Although these alkaloids meet only minor structural differences, namely the methyl group on the vindoline N atom in vinblastine replaced by a formyl group in vincristine, they significantly differ in terms of the toxicity and clinical activity spectrum. Neuropathy has often been observed following vincristine administration, while the dose-limiting toxicity for vinblastine has been carried out by myelosuppression (13).
Additionally, mixed treatment is an appealing technique for minimizing the side effects of vinca alkaloids, which are incorporated into various chemotherapy medicines to boost their results. Medications are commonly administered simultaneously as a cocktail or sequentially to optimize their therapeutic impact (14).
2. Objectives
The present study has emphasized breast cancer treatment using potential anti-cancer drugs. Here, a possible synergism has been hypothesized among taxol, vinblastine, and vincristine against the MCF-7 breast cancer cell line.
3. Methods
3.1. Chemicals
Dulbecco’s modified eagle medium (DMEM) and penicillin-streptomycin from Inoclon (Iran), and fetal bovine serum (FBS) from Gibco (USA), were responsible for providing the culture media, and other chemicals such as phosphate-buffer saline (PBS), MTT (code 1001904784) and DMSO from Sigma-Aldrich (Germany). Besides purchasing taxol, vinblastine, and vincristine from Sobhan oncology, Iran, the plastic was done from Sorfa (China).
3.2. Human Cancer Cell Line
Iranian Biological Research Center (IBRC) contributed to purchasing the human cancer cell line. The cell line, which has been employed in the present study, includes human breast adenocarcinoma (MCF-7; IBRC No. C10682). The IBRC instructions were employed for culturing the cells. DMEM was specified as the culture medium. Penicillin, streptomycin, and 10% fetal bovine serum were used to supplement the medium. Moreover, a humidified incubator at 37°C and 5% CO2 was used to maintain the cells.
3.3. MTT Assay
Trypsinizing the MCF-7 cells were conducted at 80 - 90% confluence. They were also plated into a 96-well culture dish whose density was found to be 1 × 104 cells/well overnight. The exposure of cells to different concentrations of taxol, vinblastine, and vincristine was carried out. Besides, they were combined with taxol for 48 h. The modified version of Shabani et al.'s (15) viability test of MTT, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, was done. Changing the cell medium was conducted with a fresh one (100 μL per well) after incubation. Besides, 50 μL of MTT solution (0.5 mg/mL in PBS) was added. Incubation of the plates was done for four h at 37°C. Dissolution of the formed formazan crystals was also carried out in 100 μL DMSO per well upon mixing. In order to measure the absorbance at 570 nm, a 96-well plate reader was used. The control's cell viability was presented as a percentage of cytotoxicity aligned with cell viability. The untreated cell viability was found to be 100% when all cytotoxicity was investigated. In addition, nonlinear regression via AAT Bioquest program (www.aatbio.com) was employed for estimating the IC50 values.
3.4. Experimental Design
As to the evaluation of taxol, vinblastine, and vincristine performance on the MCF-7 cell line, different concentration levels (10, 20, 40, 80, 160, and 320 µmol/mL) of each of the drugs were examined using the MTTcellproliferationassay. Based on the cytotoxicity results of the studied treatments, the synergistic effects of taxol, along with each of the vinblastine and vincristine treatments, were studied.
3.5. Synergy Testing
The Chou-Talalay method was used to assess interactions taking place among taxol, vinblastine, and vincristine on MCF-7 cells. Besides, CalcuSyn software (Biosoft, Cambridge, UK) was used for generating CI values.
3.6. Statistical Analysis
The repetition of each experiment occurred at least three times. A statistical software program (SPSS 16) was run for data analysis. Statistics through one-way ANOVA and Duncan measure were done to examine whether there were significant differences among the mean values. Results have been stated as mean ± SEM, and a P-value of less than 0.05 (P < 0.05) stands for the statistically significant difference.
4. Results
4.1. Effectsof Taxol, Vinblastine, and Vincristine on MCF-7 Cell Line
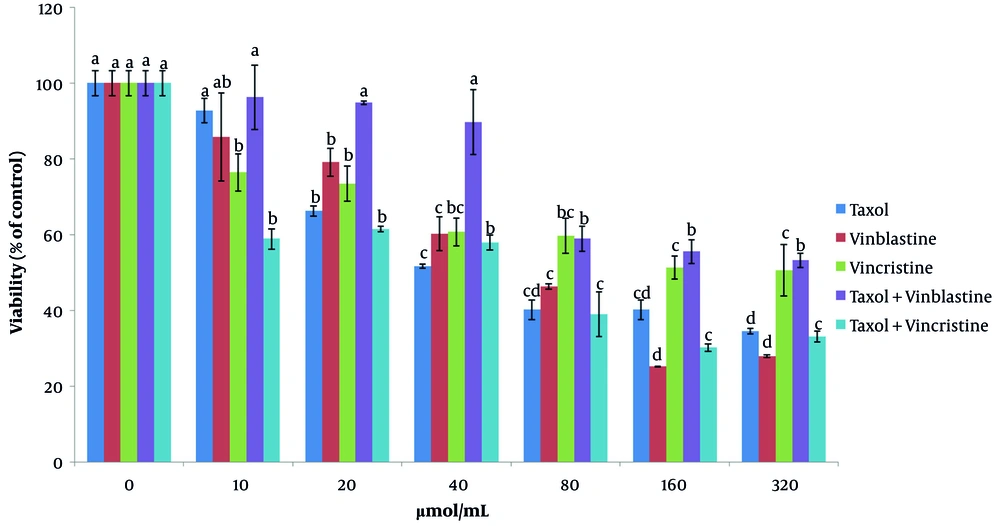
The cytotoxicity of taxol, vinblastine, and vincristine was investigated separately, and the calculation of IC50 for all drugs was conducted. To this end, MCF-7 cells were incubated with different concentrations of taxol, vinblastine, and vincristine (Figure 1). The cytotoxicity of the examined drugs was evaluated within a concentration range of 10 - 320 µmol/mL. The present results indicated that with increasing concentrations of taxol (from 10 to 20 µmol/mL), the cell viability significantly decreases after 48 h of incubation (P < 0.05). At a taxol concentration level of 80 µmol/mL, the estimation of cancer cells survival was presented as 40.17 ± 2.59. Investigating the influence of vinblastine on MCF-7 cells, it can be concluded that the survival of cancer cells (25.22 ± 0.09) significantly decreases with an increment in the concentration level up to 160 µmol/mL (P < 0.05) (Figure 1).
The results corresponding to the effect of vincristine on MCF-7 cells indicated that the survival of cancer cells at 40 µmol/mL has significantly decreased (60.67 ± 3.61) (P < 0.05).
The results obtained for the effect of the taxol + vinblastine combination on the survival of MCF-7 cells indicated that there is a significant decrease in the survival of cancer cells up to 80 µmol/mL (58.9 ± 3.38) (P < 0.05) (Figure 1).
The effect of combining various concentrations of taxol and vincristine on cancer cells indicated that the 80 µmol/mL level of concentration significantly reduces the survival of the examined cells (39.01 ± 5.8) (P < 0.05) (Figure 1).
4.2. Cytotoxicity in MCF-7 Cell Line
The examined compounds were applied to the cells for 48 h at different concentrations ranging from 10 - 320 µmol/mL. The MTT test assessed the cytotoxicity, and the values of IC50 were also estimated. Table 1 presents the findings of 50% inhibitory features associated with the investigated experiments. The best inhibitory effects are associated with the taxol + vincristine, taxol, and vinblastine treatments with concentrations of 41.45, 64.46, and 67.12 µmol/mL, respectively.
| Treatments (µmol/mL) | |||||
|---|---|---|---|---|---|
| Cell Line | Taxol | Vinblastine | Vincristine | Taxol + Vinblastine | Taxol + Vincristine |
| MCF-7 | 64.46 | 67.12 | 239.51 | 236.71 | 41.45 |
The Investigated Compounds' Cytotoxicity Against the MCF-7 Cell Line
4.3. Synergistic Effect of Taxol
When loading of the surviving fractions into CalcuSyn software was carried out, CI (combination index), which is derived from the Chou and Talalay method for assessing synergy, antagonism, or addition, was calculated. Results revealed that a synergistic reaction was recorded between the taxol and vincristine combination of drugs (CI < 1).
5. Discussion
Today, one way to increase the anti-cancer effect is by combining chemotherapy with natural compounds, which can lead to improving the therapeutic window and reversing resistance to the drug (16). The present results indicated that taxol at a concentration of 64.46 µmol/mL meets 50% inhibitory properties on MCF-7 cells. The calculated IC50 values were higher than those of vinblastine (67.12 µmol/mL) and vincristine (239.51 µmol/mL). Cell cycle arrest and apoptosis might result from exposing cells to taxol, which might lead to effects that align with the different concentrations of drugs and employed tissues and/or cell lines (8). A number of researchers have acknowledged that taxol can activate various signal transduction pathways. For instance, (1) the activation process of c-jun N-terminal kinase (JNK) can be carried out by taxol using the Ras and apoptosis signal-regulating kinase (ASK1) pathways. JNK and p38 can also be activated by ASK1, which is a MAP kinase. When there is TNF-K treatment, apoptotic cell death can be induced by ASK1; (2) when extracellular signal-regulated kinases (ERK) are to be activated, the process is induced by taxol. Taxol-treated RAW 264.7 cells were also significant in forming anShc/Grb2 complex and Tyrosine phosphorylation of Shc; (3) p38 tyrosine phosphorylation results from taxol (9). Additionally, several investigations have indicated that the taxol resistance in breast cancer cells might be due to the increased levels of βIII isotypes (17). The major cause of the reduction of clinical effects can be attributed to the achieved resistance to taxol, unfortunately (16). In this regard, the present study examined the effect of taxol along with vinca alkaloids. According to the results obtained, the best inhibitory effect is related to taxol + vincristine treatment with concentrations of 41.45 µmol/mL. The use of combination therapy has been shown to play a key role in the therapy of cancer. It also results in a more therapeutic improvement than the monotherapeutic modality (2). Possessing the ability to induce specific changes in DNA, such as micronuclei and oxidative, but not others (i.e., SCEs) appears to be the potential of vinca alkaloids. This ability can be attributed to the vincaalkaloid’s performance characteristics, which is in alignment with apoptosis activation. The activation process proceeds when there is interference with spindle fiber formation and mitochondrial function. Furthermore, changes in models, doses, and duration regarding the treatment of the drug can be caused by improper variations occurring in genotoxicity, which are aligned with vincristine in vitro cultured cell methods and mammalian models (11). The side effects of vinca alkaloids can be reduced by a promising approach known as combination therapy. In other words, a combination occurs when other chemotherapy drugs are involved, which can increase the potential for antitumor treatment. Administering the drugs occurs concurrently as a cocktail, or it might take place sequentially to enhance their therapeutic effectiveness (14).
Being resistant to chemotherapy can be a significant hindrance to therapeutic success. There appears to be significant demand to seek more effective techniques for therapy (16). Cell viability measurement was conducted to specify the most synergistic effect for the different combinations. Results revealed that, according to the measured CI, synergistic performance occurred between taxol and vincristine.