1. Background
Doxorubicin (Dox), sold under the brand name Adriamycin, is a chemotherapy anthracycline antibiotic used to treat many cancers, including breast cancer, bladder cancer, Kaposi’s sarcoma, lymphoma, and acute lymphocytic leukemia (1). It exerts its anti-cancer effects primarily through direct interference with the function of DNA (2). Yet, it has been shown to negatively affect non-target healthy tissues. It inhibits the DNA-dependent production of RNA by placing itself between two pairs of DNAs and opening the chains, thereby exerting its toxic effect on the cell. Dox also prevents protein synthesis and causes apoptosis (3). Production of reactive oxygen species (ROS) and stimulation of apoptotic cell death play pivotal role in the toxicity of various tissues, such as the heart and liver tissues (4).
Although there is currently no optimal solution for preventing Dox-induced liver cytotoxicity, consistent training has been perceived by the corresponding academic circle as a viable candidate. Zolfagharzadeh and Roshan (5) found that moderate-intensity aerobic training could balance oxidation and anti-oxidation factors in the liver tissue of Dox-injected male rats, which was a protective approach. Dadban Shahamat et al. (6) reported that continuous training (CT) significantly reduced the apoptotic index of the ratio of pre- to anti-apoptotic mitochondrial proteins (Bax/Bcl2) in the liver tissue of elderly rats exposed to Dox induction. Furthermore, Azizi et al. (7) determined that moderate-intensity aerobic exercise had a preventive effect on cardiac tissue apoptosis in rats exposed to Dox induction.
Several researchers have also suggested using herbal supplements as an effective solution for dealing with the given problem. One of the most prevalent antioxidant supplements for curing various diseases is crocin (Cr). Crocin is a water-soluble carotenoid found in the flowers of crocus and gardenia plants, and is responsible for the main color of saffron. Crocin has anti-cancer properties (anti-cell proliferation and pro-apoptotic) useful in dealing with malignant cells and, at the same time, it bears antioxidant, anti-hyperlipidemic, as well as anti-atherosclerotic and functions as an agent for cardiac, liver, and nerve tissue protection. Evidence suggests that the antioxidant properties of Cr affect apoptosis’s internal and external pathways (8). Hoshyar et al. (9) have documented that Cr administration increases apoptosis in human gastric adenocarcinoma (AGS) cancer cells and inhibits cancer cell progression. Elsherbiny et al. (10) have also reported that Cr nasogastric feeding reduces the caspase-3 expression, an indicator of cardiac tissue apoptosis in rats exposed to Dox toxicity. Taking into account the findings, therefore, it has been confirmed that Cr plays a significant role in protecting cells and healthy tissues against cancer by inducing and inhibiting apoptosis.
2. Objectives
Considering the alteration caused by Dox induction and its effect on liver tissue function, therefore, this study aimed to examine the anti-apoptotic effect of adopting the eclectic approach of CT and Cr supplementation on liver tissue in Dox-induced male rats.
3. Methods
3.1. Animals
In this experimental study, a post-test design was adopted by using 40 adult male Wistar rats aged 8 weeks, weighed approximately 220 ± 20, and purchased from the breeding research laboratory of Ahvaz Jundishapur University of Medical Sciences. They were preserved under standard research conditions (i.e., the temperature of 25 ± 2, relative humidity of 50%, the dark-light cycle of 12: 12 hours, and free access to water and food) after being transferred to the histology department laboratory of Jundishapur University of Medical Sciences and kept there for seven days for adaptation purposes. Rats failing to comply with the standard requirements were excluded from the laboratory. It should be also noted that the guidelines of the National Ethics Committee in Biomedical Research, the Principles of the 2008 Helsinki Declaration, and the Code of Ethics (IR.IAU.M.REC.1398.013) for animal physical training research were followed in this study.
3.2. Study Groups and Treatment of Animals
The animals were randomly divided into five groups, each of which including 8 rats, as follows:
(1) Healthy control group
(2) Dox
(3) Dox + Cr
(4) Dox + CT
(5) Dox + CT + Cr
Two mg/kg of rat body weight of Dox hydrochloride (Belgian Ebewe Company) was diluted with normal saline and was subcutaneously injected seven times (cumulative dose 14 mg/kg) on Fridays (48 hours after the last training session and 24 hours before the next exercise session) (11). All injections were performed at 10:00 a.m., and the healthy control group was injected 0.9% normal saline subcutaneously to equalize the other groups.
Crocin supplement (Sigma-Aldrich co, USA) with pharmacological grade and purity of 98% was diluted in a 1-gram vial with normal saline, and 10 mg/kg rat’s body weight was administered orally at 10 a.m. after the training sessions for eight weeks. Other saline groups were given the same amount of Crocin supplementation through a nasogastric tube (10).
3.3. Exercise Training Procedure
Before performing the main training protocol, a light training program including 10 sessions of walking and running at a speed of 8 to 10 meters per minute with a zero-degree slope for 5 to 10 minutes in two weeks (10 days) was implemented in order for enabling the rodents to familiarize themselves with performing on rotating bars. The continuous training program consisted of three parts: Warm-up, cool-down (5 minutes 40 to 50% Vmax), and the main exercise (Table 1). The exercise program was carried out for eight weeks, and five sessions on a weekly basis at 8 am (12).
| Factor | Warm-Up | Intense Training | Cool-Down | |||
|---|---|---|---|---|---|---|
| Training intensity (% Vmax) | 40% - 50% | Week 1 | Week 2 | Week 3 | Week 4 - 8 | 40% - 50% |
| 60% | 65% | 70% | 70% | |||
| Duration (min) | 5 | 16 | 24 | 32 | 40 | 5 |
3.4. Animals’ Dissection and Sampling
Rats were anesthetized 5 minutes after following 12 hours of fasting and 48 hours of the last training session as well as oral administration of Cr supplementation by performing intraperitoneal injection of 10% ketamine (50 mg/kg body weight) and 2% xylazine (10 mg/kg body weight). Liver samples were isolated by a specialist and soaked in 10% formalin for 48 hours. Then they were cut into longitudinal and transverse slices. The samples were stored in microtubes for tissue storage at -80°C in liquid nitrogen for measurement.
3.5. TUNEL Staining
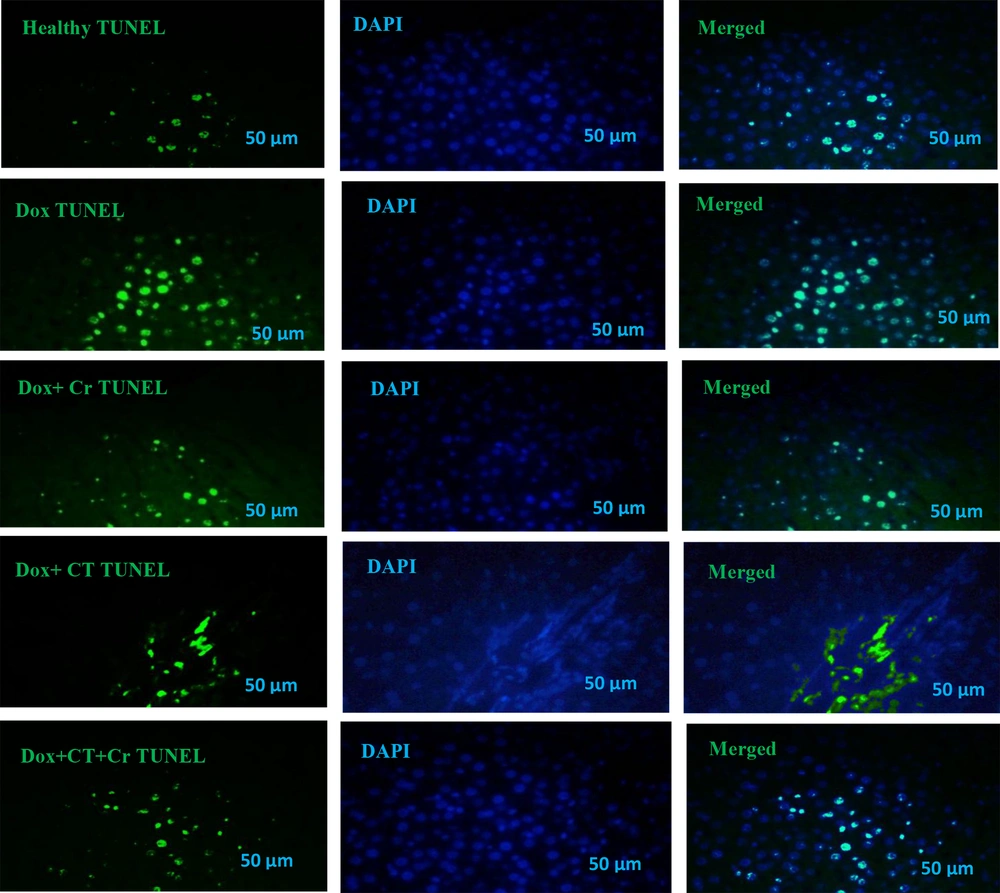
Liver tissue sections were incubated with proteinase K, washed in phosphate saline buffer (PBS), incubated with permeabilization solution, blocking buffer, and washed twice with PBS. Tissue sections were stained with terminal deoxynucleotidyl transferase and fluorescein isothiocyanate-dUTP (TUNEL) (Roch-11684817910) for 2 hours at 37°C to identify the number of apoptotic nuclei (green dots). Tissue sections were also stained with 0.1 g/mL 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich-D9542, USA) for 5 minutes, and the nuclei were detected by fluorescent microscopy (Labomed, USA) at 454 nm with a magnification of 400x. DAPI staining (blue dots) is a general dye that shows the population of apoptotic and non-apoptotic cell nuclei is considered, and TUNEL staining (green dots) determines the nucleus of apoptotic cells. And basically, the overlap of these two colors is considered the percentage of apoptotic cells compared to the total cells for analysis.
3.6. Statistical Analysis
Shapiro-Wilk test was used to examine the normal distribution of the data. In addition, an independent t-test and two-way analysis of variance were performed using the Bonferroni post hoc test in order for testing the research hypotheses. All statistical operations were performed using SPSS26 software, and statistical significance was set at P < 0.05.
4. Results
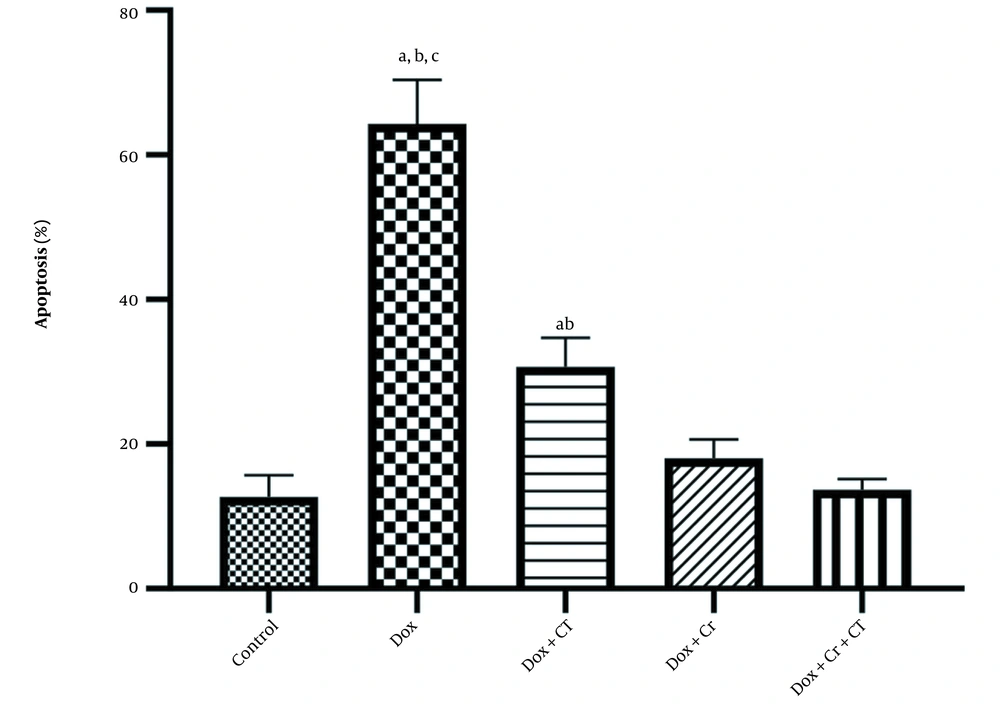
Evaluation of TUNEL staining compared to DAPI staining was conducted, and the following percentages were determined for liver cell apoptosis in different experimental groups: Healthy control (12.67% ± 3.05), Dox (64.33% ± 6.02), Dox + Cr (18% ± 2.64), Dox + CT (30.67% ± 4.04), Dox + CT + Cr (13.67% ± 1.52) (Figure 1).
The results of independent t-test showed that doxorubicin caused a significant increase in hepatocyte apoptosis compared to that in the healthy control group (t = -13.24, P = 0.0001). Furthermore, the results of two-way ANOVA indicated that the apoptosis of rat liver cells caused by Dox induction was significantly reduced in all intervention groups (P = 0.0001; Table 2).
| Parameter | Comparison of Doxorubicin-Control | Two-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|
| Continuous Training | Crocin | Crocin × Training | ||||||
| T | P | F | P | F | P | F | P | |
| Apoptosis | -13.24 | 0.0001 | 69.87 | 0.0001 | 194.08 | 0.0001 | 41.63 | 0.0001 |
In addition, liver apoptosis level in Dox + CT + Cr (P = 0.004; Figure 2) and the Dox + Cr (P = 0.02; Figure 2) was significantly lower than that in the Dox + CT group. However, no significant difference was observed between the two groups receiving Crocin (P = 1; Figure 2).
The effect of continuous training and crocin on the level of apoptosis in the experimental groups based on the mean ± SD: A, Significant differences with doxorubicin (Dox) + crocin (Cr) + continuous training (CT); B, Significant differences with Dox + Cr; C, Significant differences with Dox + CT; P < 0.05
5. Discussion
The current study aimed to examine the effects of Dox on the apoptosis of male rat liver cells adopting the TUNEL method. To this end, the anti-apoptotic effects of CT and Cr supplementation were evaluated separately and collectively. The results of the present study showed that Dox increased hepatic cell apoptosis. Numerous studies have been performed in this regard. Moradi et al. (13) found that Dox increased the expression of the Bax apoptotic and Bax/Bcl2 ratio, and decreased the expression of the Bcl2 anti-apoptotic in liver tissue compared to those of the healthy controls. Patel et al. (14) also reported that doxorubicin increased p53 and cytochrome C expression, decreased Bcl-xl expression, and increased apoptosis in rat liver tissue.
Considering the consistency of the present results with those of the previous studies, seemingly anthracyclines (including Dox) not only destroyed mitochondrial respiration and bioenergy by increasing free radicals but also led to swelling and rupture of the inner mitochondrial membrane, eventually releasing Bcl2 family proteins that formed non-selective channels in the membrane and activate apoptotic pathways (15). On the other hand, the p53 tumor suppressor transcription factor is phosphorylated on serine/threonine-specific residues in response to cellular stresses such as oncogene activation, hypoxia, and DNA damage. Transcription of p53 apoptotic pathways is transmitted to the mitochondria, interacting with the anti-apoptotic protein Bcl-xl to release cytochrome C into the cytosol (16).
Our study results showed that moderate-intensity CT for eight weeks reduced doxorubicin-induced hepatocyte apoptosis in male rats. In line with the findings of our study, Zou et al. (17) performing the TUNEL assay also found that 12 weeks of endurance swimming training reduced apoptosis in hepatocytes of zebrafish with nonalcoholic fatty liver disease (NAFLD). Alihemmati et al. (18) demonstrated that interval aerobic training mitigated pre-apoptotic indices of Bax/Bcl2 ratio and Bax levels and caspase-6 while increasing Bcl2 levels. The results of the TUNEL assay further revealed that their routine also had the potential to reduce the number of apoptotic cells in the heart tissue of Dox-induced rats. In contrast, Kazemi and Mirza-zade (19) reported that continuous 6-week aerobic training caused an increase in apoptotic biomarkers such as caspase 3 and 9 and, therefore, was effective in reducing breast cancer cells in female rats. Although their results were inconsistent with findings from later studies, they confirmed the effectiveness of physical activity in combating cancer through a different mechanism. Previous studies have also shown that exercise affects cancer tissues by inhibiting cell growth through increasing apoptotic pathways but reducing the risk of oxidative stress damage and the inflammatory pathway in healthy tissues due to mechanical stress and increases antioxidant defense in the absence of over-training (20).
This study revealed that administering 10 mg oral Cr supplementation in rats reduced Dox-induced hepatocyte apoptosis. Previous studies have determined that Cr reduces caspase-3 and activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) of heart tissue exposed to hyperhomocysteinemia-induced damage (21). Chu et al. (22) found that Cr stimulated the heart cells of Dox-induced rats by regulating the apoptotic proteins Bax, Bcl2, and caspase-3 and by improving the expression of TLR-2, NF-κB. Furthermore, Veisi et al. (23) reported that Cr could eliminate cancerous cells by activating caspase-8 apoptotic pathways, p53 expression, reduced Bcl2 as well as Bax/Bcl2 ratios, and surviving cyclin D1 proteins. Considering the consistency of the previous findings with our results regarding Cr’s capacity to prevent apoptosis-induced cytotoxicity in healthy tissues, NF-κB activation by protein kinase B (Akt) was responsible for the survival and transcription of cell growth genes (24). Cr also prevents apoptosis by regulating the anti-apoptotic proteins Bcl2, and Bcl-xl (25).
As far as the primary contribution of our study is concerned, it indicated that the combination of moderate-intensity CT and oral Cr administration reduced hepatocyte apoptosis in Dox-induced male rats. Previous studies have documented that training and Cr decreased Bax expression and Bax/Bcl2 ratio while increasing Bcl2 in testicular and skeletal muscle tissues of male rats (26, 27). According to the findings from previous studies, therefore, it is implied that the combination of CT and Cr administration is associated with reducing oxidative stress and enhancing the anti-apoptotic proteins of the mitochondrial membrane of the Bcl2 family. This, in turn, prevents the release of cytochrome C and the formation of apoptosis in the cytosol, thereby inhibiting the caspase pathway of apoptosis (28). Our study also indicated a further deceleration of Dox-induced apoptosis of liver tissue by administrating Cr orally compared to CT (Table 2 and Figure 2). Studies have shown that oral Cr is hydrolyzed to crocetin in order to facilitate absorption in the intestine, which enters the liver quickly and in large amounts (8), which may justify its effectiveness. Similarly, the lack of significant differences between the Cr and training group with the Cr group alone can be justified by the fact that sometimes antioxidant supplementation can stop the adaptations resulting from physical activity (29). According to our study results, using Cr and CT may not have produced more favorable synergistic effects than using Cr alone.
Doxorubicin causes apoptosis and tissue toxicity by producing ROS and inactivating the survival, growth pathways, and cell proliferation in cancerous and healthy tissues; however, these factors were not measured in our study, which was the limitation of this study. Therefore, it was recommended that future studies should be conducted in order to investigate the effect of CT and Cr supplementation on ROS production such as mtDNA and different cell survival and growth signaling pathways such as NF-κB, C-jun, TNF-α, and Akt and also, and apoptotic biomarkers such as caspases, cytochrome C, apoptotic inhibitory proteins, Bax, Bcl2, Bcl-xl.
5.1. Conclusions
Clinical findings of this study revealed that CT and Cr reduced hepatic cell apoptosis and hepatotoxicity induced by Dox induction. However, Cr consumption was found to be more effective than CT. Therefore, intervention agents may have been used as a non-pharmacological treatment for dealing with the side effects of chemotherapy drugs.