1. Background
Hepatocellular carcinoma (HCC) is a prevalent cancer and the third cause of cancer death in the world (1). The highest incidence of HCC is seen in Asia, and according to estimates, there are 782,000 new cases in the world (2). The current treatment for patients with HCC is chemotherapy and radiotherapy, followed by surgery (1). However, resistance to chemotherapeutic drugs has been increased, and, therefore, the efficiency of chemotherapy has decreased (3). To address this issue, growing research attention has been given to various herbal medicines and other natural compounds recently in order to explore their anti-cancer activities and develop novel agents for treating HCC and other human cancers (4).
Previous studies have reported the potential anticancer activities of various herbal medicines (5, 6), and ample evidence has confirmed the presence of various compounds with antitumor effects in the extracts and essential oils from various herbal medicines (5, 7). The Satureja genus is composed of more than 200 species distributed in the Mediterranean and Asia. The aerial parts of Satureja species are used to treat several diseases, including urinary tract infections, wounds, gastroenteritis, and diarrhea (8). In addition, extraction and essential oils of Satureja species have been reported to show several biological activities such as antibacterial, antifungal, anti-inflammatory, antidiabetic, and antioxidant activities (9). Satureja sahandica is an important species of the Satureja genus, which, according to previous studies, is rich in gamma-terpinene, carvacrol, p-cymene, and thymol (10). The antimicrobial and antioxidant effects of S. sahandica have also been confirmed by several studies (9, 11). Other studies have reported the total phenolic content, nitric oxide radical scavenging, and ferrous ion chelating of S. sahandica (12). However, the anti-proliferative and anticancer effects of S. sahandica extraction on human cancer cells have received no research attention so far.
Layered double hydroxides (LDHs) are a host matrix with a two-dimensional (2D) structure whose various organic (e.g., benzoates, sulfonates, and carboxylates) and bimolecular anions (e.g., enzymes, amino acids, and drugs) are able to intercalate in LDHs interlayers (13, 14). Therefore, LDHs can be applied as drug delivery carriers (15).
2. Objectives
So far, a limited number of studies have investigated the synergetic effects of S. sahandica in combination with LDH Zn3Al nanoparticles on cancer cells. This study aimed to examine the anti-proliferative effects of S. sahandica in the co-administration of LDH on HepG2 cancer cells by bio-conjugating the plant extraction as a phytochemical structure to Red 27-Zn3Al LDH nanosheets solution.
3. Methods
3.1. Syntheses of Red 27-Zn3Al-LDH Nanosheets
The acid red 27 (0.001 mol, 0.6044 g) was dissolved in NaOH (0.1 M, 20 mL) and then was added to Al(NO3)3.9H2O (0.001 mol, 0.375 g) and Zn (NO3)2.6H2O (0.003 mol, 0.89235g) solutions in a nitrogen atmosphere, adjusted to pH 9 by HCl or NaOH, and heated for 72 hours in 60°C. The obtained solution was centrifuged and dehydrated in vacuum at room temperature. Finally, the obtained red 27-Zn2Al-LDH was stirred with formamide solution (100 cm3) for 24 hours to produce exfoliated Zn3Al-LDH nanosheets.
3.2. Preparation of Plant Extraction
Satureja sahandica was collected from Maragheh City, and the powdered S. sahandica was mixed with chloroform at the ratio of 1: 10 (w/v) for two weeks at an ambient temperature. Then the obtained extracts were filtered and concentrated using a rotary instrument. Finally, the extracts were dried and stored at 4°C.
3.3. Cancer Cell Culture
The hepatic cancer cell line HepG2 was purchased from Pasteur Institute, Tehran, Iran. The cells were grown by RPMI media supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin antibiotics and were incubated in a standard condition (i.e., 37°C in the humidified atmosphere 5% CO2).
3.4. Cancer Cell Viability Assay
The hepatic cancer cell line HepG2 was seeded in 96-well plates and incubated overnight. The cancer cells were treated with the S. sahandica extraction and LDH nanosheets for 24 and 48 hours. After this period, the old media was discarded, and then a 200 μL fresh culture media containing 50 μL of MTT solution was added to each well and incubated for four hours in the standard condition. Old culture media was substituted with 50 μL of dimethyl sulfoxide (DMSO) and then incubated for 30 minutes in the standard condition. The optical densities (OD) of the wells were identified at 578 nm wavelength using an ELISA reader.
3.5. Cancer Cell Morphology Assay
The hepatic cancer cell line HepG2 was seeded in 6-well plates and incubated overnight. Then the cancer cells were treated with S. sahandica extraction. Finally, all morphological alterations in the treated cancer cells were searched using an inverted phase-contrast microscope (Optica, Iran).
3.6. Trans-well Migration Assay
The hepatic cancer cell line HepG2 was seeded in the upper trans-well chamber containing RPMI medium (serum-free) and treated with the S. sahandica extraction. In addition, RPMI medium (with 10% FBS) was added to the bottom trans-well chamber. After incubation for 20 hours at 37°C, the migrated cancer cells were fixed and stained by 0.1% crystal violet in the bottom chamber. The migration of the cancer cells was monitored in different random fields.
3.7. Apoptosis Qualifying
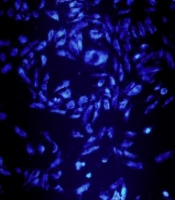
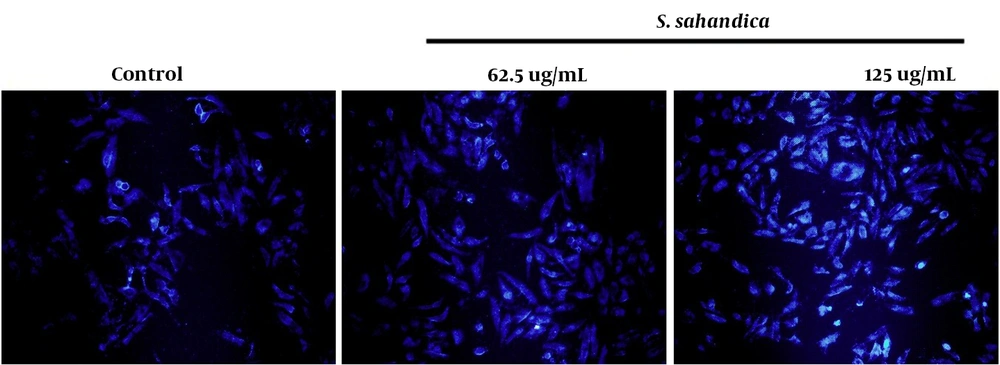
The 4,6-diamidino-2-phenylindole (DAPI) staining method was adopted to perform an apoptosis qualification assay. The hepatic cancer cell line HepG2 was seeded in 6-well plates and incubated overnight. The cancer cells were treated with S. sahandica extraction. The cells were fixed with paraformaldehyde (3wt%) and permeabilized by Triton X-100. Finally, the cells were stained with 300 ng mL-1 DAPI and monitored in different random fields.
3.8. Apoptosis Quantification
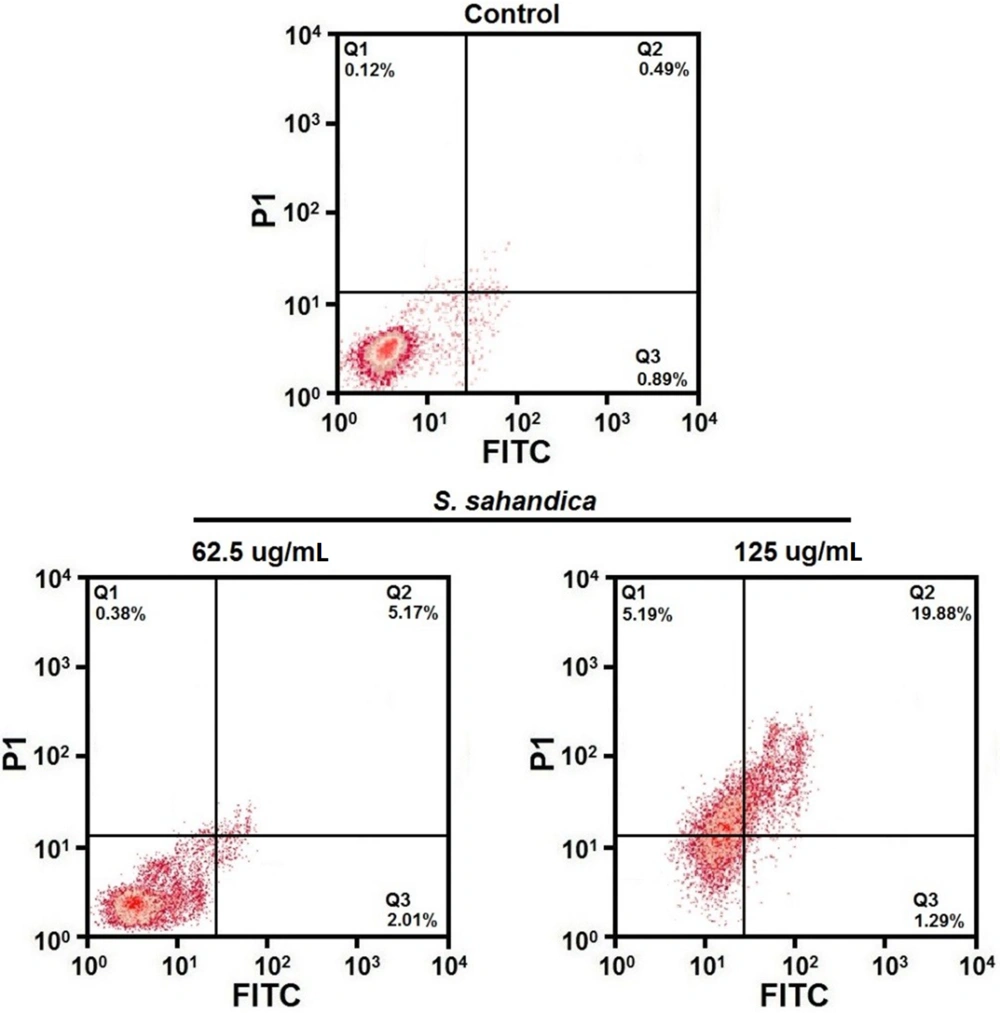
The hepatic cancer cell line HepG2 was seeded in a 96-well plate and incubated overnight. Then, the cancer cells were treated with S. sahandica extraction. The cells were resuspended and incubated with 5 mL annexin isothiocyanate V-fluorescein (FITC), 10 mL propidium iodide (PI), and 400 μL binding buffer. Finally, the apoptosis percentage in the cells was investigated by flow cytometry.
3.9. Apoptosis-Related Genes Expression Assay
The hepatic cancer cell line HepG2 was seeded in 6-well plates and incubated overnight. The cancer cells were treated with S. sahandica extraction. Total RNA was extracted by TrizoL agent. The oligo-dT primers were used to reverse the transcription of 1 μg total RNA into cDNA. The expression of apoptotic genes was evaluated using qRT-PCR. The used primer’s characteristics and sequences are shown in Table 1. The reactions were performed in a 20 μL total volume (cDNA (1 μL), each primer (0.5 μL), and PCR pre-Mix (18 μL)). The expression of ACTB gene (β-actin) was evaluated as endogenous control.
| Gene and Primers Sequences | Products Size (bp) |
|---|---|
| BAX | 108 |
| F-GGTTGTCGCCCTTTTCTA | |
| R-CGGAGGAAGTCCATGTC | |
| BCL2 | 88 |
| F-GATGTGATGCCTCTGCGAAG | |
| R-CATGCTGATGTCTCTGGAATCT | |
| ACTB | 93 |
| F-GCGAGAAGATGACCCAGAT | |
| R-GAGGCGTACAGGGATAGC |
3.10. Statistical Analysis
In this study, all experiments were repeated three times, and the results were presented as mean ± standard deviation (SD). The statistical analysis of the data was conducted by performing ANOVA, Tukey, and Student’s t-test and using Graph Pad Prism software. The difference with P < 0.05 was reported as statistically significant.
4. Results
4.1. Cancer Cell Viability
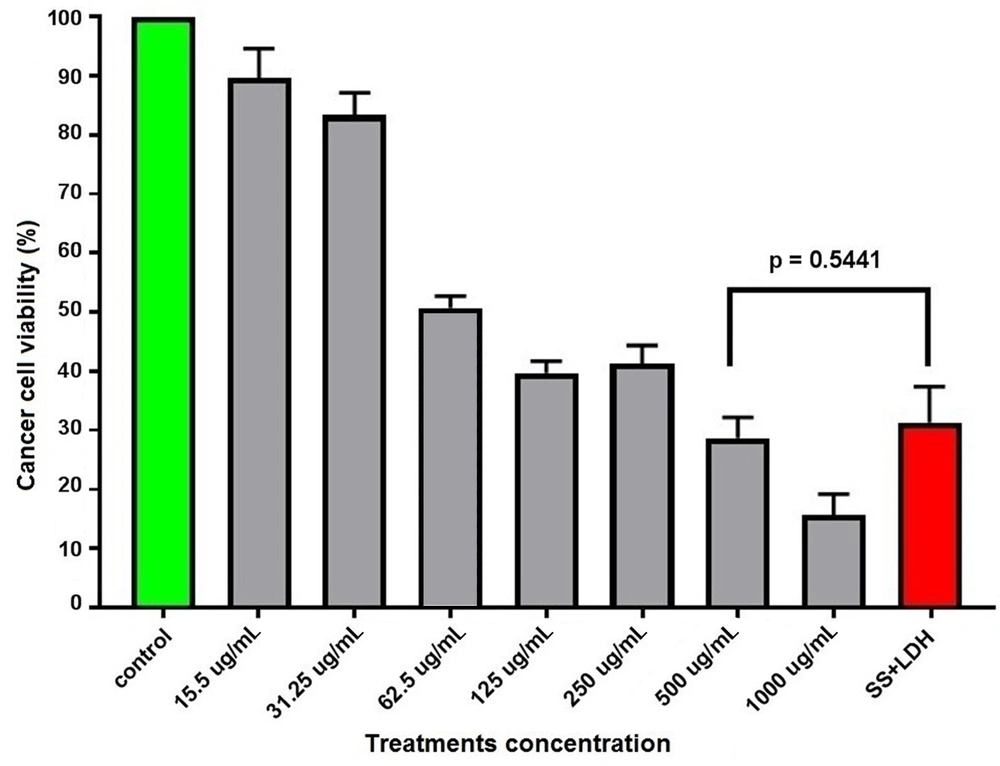
According to the study results, the S. sahandica extraction had anti-proliferative effects and inhibited the proliferation of the hepatic cancer cell line HepG2. The results were also suggestive of the antiproliferative activity of S. sahandica extraction. IC50 of the S. sahandica extraction on cancer cells was 62.5 μg/mL after 24 hours. Moreover, the antiproliferative activity of the S. sahandica extraction decreased in combination with LDH nanosheets. However, no significant differences were observed between S. sahandica extraction and LDH nanosheet combination and monotherapy (Figure 1).
4.2. Cancer Cell Morphology
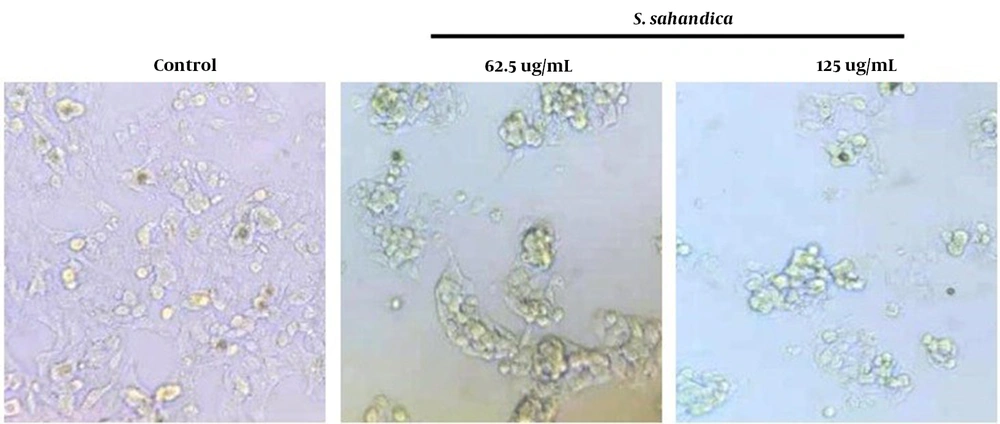
Several alterations were detected in the morphology of the hepatic cancer cell line HepG2 treated with the S. sahandica extraction, which was indicative of the cancer cell death through apoptosis. The morphological alterations include cell size decrease, fragmented nuclei, cell shrinkage, and membrane damage. These morphological alterations in the cancer cells treated with S. sahandica extraction were time- and concentration-dependent (Figure 2).
4.3. Cancer Cell Migration
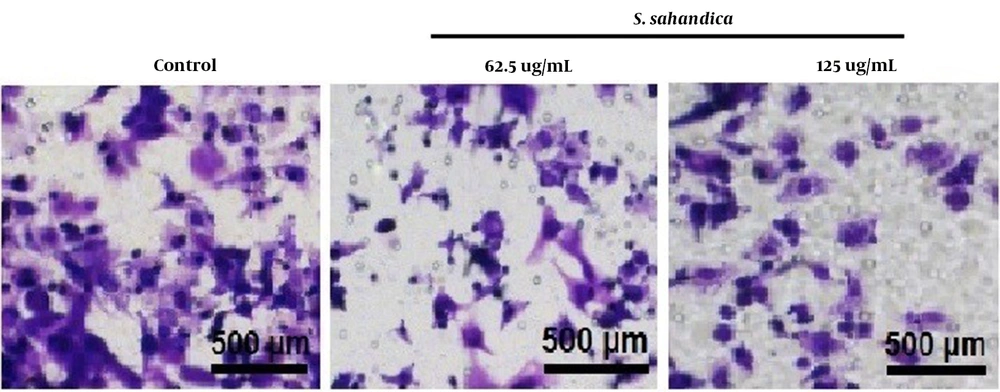
The trans-well assay indicated that the migration of cancer cells treated with the S. sahandica extraction was significantly decreased in a concentration-dependent manner (Figure 3).
4.4. Cancer Cell Apoptosis
Apoptosis qualification demonstrated a fragmented nuclear and chromatin condensation in HepG2 cancer cells (Figure 4). Apoptosis quantification showed an increased rate of apoptosis in the treated HepG2 cancer cells. The total percentage of apoptosis in the treated cells with 125 μg/mL S. sahandica extraction was 21.17% (Figure 5).
4.5. Apoptosis-Related Genes Expression
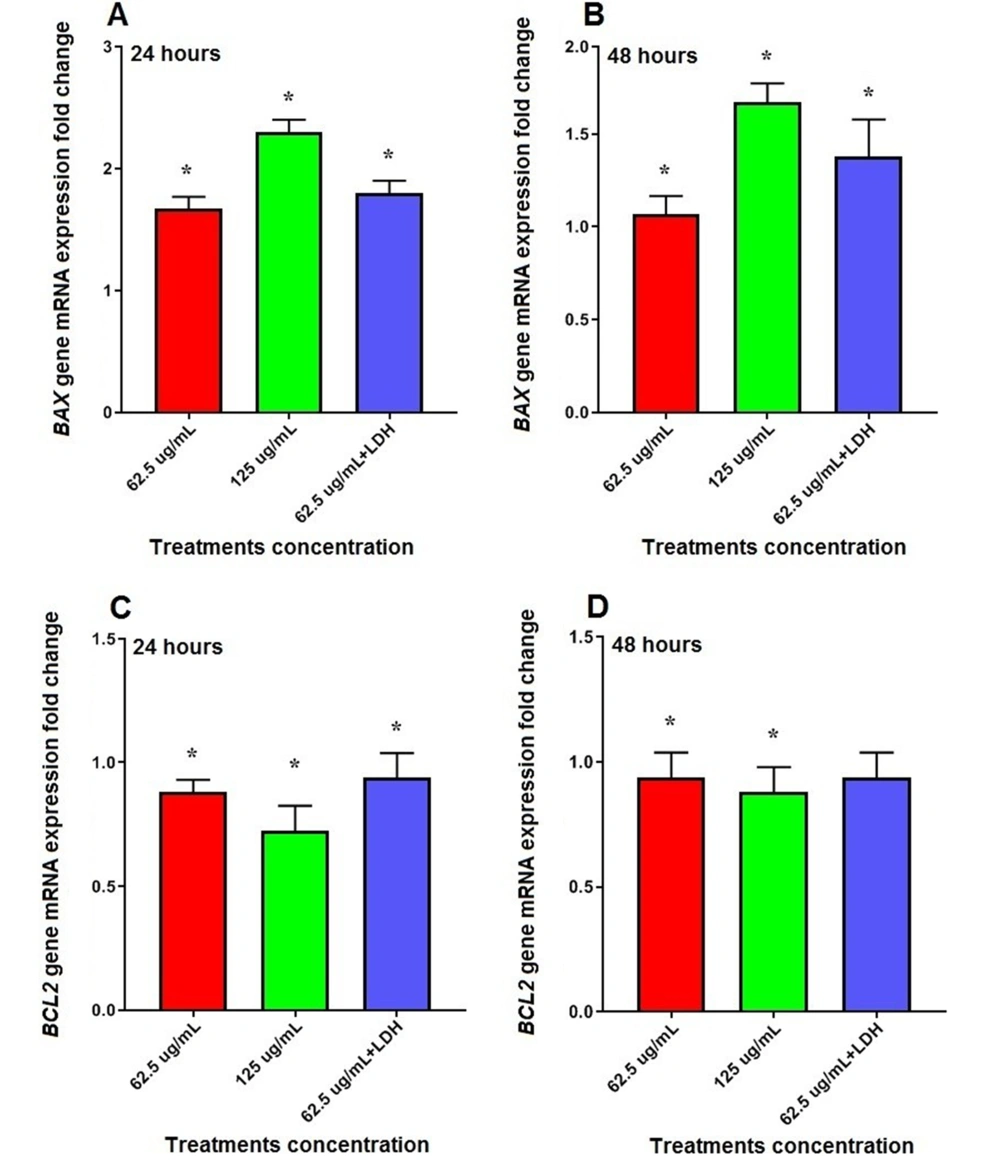
According to our results, mRNA expression of BAX was significantly increased by 1.67 (62.5 μg/mL) and 2.30 (125 μg/mL) fold in hepatic cancer cell line HepG2 treated with the S. sahandica extraction after 24 hours (P < 0.01). However, the expression of this gene increased 1.90-fold in cancer cells treated with the S. sahandica extraction in combination with LDH nanosheets (Figure 6A).
Moreover, the treatment of cancer cells with S. sahandica extraction significantly increased mRNA expression of BAX by 1.06 (62.5 μg/mL) and 1.67 (125 μg/mL) fold after 48 hours (P < 0.01). However, the expression of BAX increased 1.38-fold in cancer cells treated with the S. sahandica extraction in combination with LDH nanosheets (Figure 6B).
In addition, mRNA expression of BCL2 was significantly decreased by 0.88 (62.5 μg/mL) and 0.72 (125 μg/mL) fold in cancer cells treated with S. sahandica extraction after 24 hours (P < 0.01). However, the expression of BCL2 decreased 0.93-fold in cancer cells treated with S. sahandica extraction in combination with LDH nanosheets (Figure 6C).
Moreover, the treatment of cancer cells with S. sahandica extraction significantly decreased 0.94 (62.5 μg/mL) and 0.88 (125 μg/ml) fold in mRNA expression of BCL2 after 48 hours (P < 0.01). However, the expression of BCL2 decreased 0.93-fold in cancer cells treated with S. sahandica extraction in combination with LDH nanosheets (Figure 6D).
5. Discussion
Various natural products and plants have been widely used for treating different kinds of diseases for over 3500 years (16). Modern pharmacology has recently paved the way for developing bioactive compounds from various medicinal herbs in order to treat diseases - human cancers, for example (17, 18). Satureja sahandica is a common medicinal plant with antioxidant and antitumor effects (10).
In the present study, the application of S. sahandica extraction was found to result in cancer cell death. Yousefzadi et al. demonstrated the cytotoxic effects of S. sahandica on the colon adenocarcinoma, breast adenocarcinoma, and choriocarcinoma cells (10). Sadeghi et al. reported that the Satureja intermedia caused cancer cell death, such as esophageal squamous cell carcinoma and bladder carcinoma cell line (19). Kundakovic et al. revealed the Satureja montana cytotoxic effects on breast cancer, epithelial cervical cancer, and colon cancer cell lines (20). The results of the above-mentioned studies were consistent with our study findings, confirming the cytotoxic and anti-proliferative effects of Satureja species on the cancer cell lines (e.g., breast, colorectal, lung, cervix, and bladder).
Moreover, the antiproliferative activities of the S. sahandica extraction and LDH nanosheet combination were evaluated in our study, and it was observed that LDH nanosheets decreased the anti-proliferative activity of S. sahandica. This may have been attributed to the interaction between extraction and LDH components in the treated HCC cells. Recently, LDH nanosheets have received particular attention in biomedical studies due to their interlayer anionic capacity and unique structure (21). Therefore, LDH nanosheets are applied for the drug delivery targeted into LDHs interlayer anion exchange. Evidence has suggested that LDH nanosheets are ideal candidates for performing cancer therapy (21, 22). In a study by Sun et al., it was discovered that the loaded indocyanine green in LDH nanosheets may have proved effective in the chemotherapy of breast cancer cells (23). In another study by Dragoi et al., it was revealed that the loaded fluorouracil in LDH nanosheets may have been a promising compound for anti-tumor drug delivery (24). However, the above-mentioned results were not in agreement with our study findings, which may have been due to the difference in synthesis presses of LDH nanosheets.
There are several alterations in the morphology of the HCC cells treated with S. sahandica extraction. These alterations include fragmented nuclei, decreased cell size, cell shrinkage, and membrane damage. The presence of various chemical compounds, including aldehyde, ethylic alcohol, acetate, and valeric, can cause morphological alterations in treated cancer cells (25, 26).
Apoptosis induction is one of the most crucial strategies for treating cancer patients with chemotherapeutic agents (27). In this study, an apoptotic percentage was significantly enhanced in HepG2 cells treated with S. sahandica extraction, indicating the effects of S. sahandica extraction on the induction of apoptosis in cancer cells. A similar study by Seyedan et al., however, found that Satureja khuzistanica essential oil prevented the doxorubicin-induced apoptosis through extrinsic and intrinsic mitochondrial pathways (28). Another study by Asadipour et al. reported that the extraction of Satureja bachtiarica produced anti-leukemic effects through the induction of apoptosis (29).
Bcl2 family members, in particular, are commonly involved in the regulation of the mitochondrial or intrinsic apoptosis pathway (30). In this regard, our study results showed that S. sahandica significantly upregulated BAX and downregulated the BCL2 expression in HepG2 cells. These genes are involved in the regulation of the apoptosis process. A higher expression of BAX causes apoptosis induction and increases cell death. A higher expression of BCL2, on the other hand, inhibits apoptosis and causes cell survival (4). Therefore, the regulation of BAX and BCL2 gene expression plays a strategic role in cancer therapy (31, 32). A study by Esmaeili and Farimani suggested that the S. sahandica extraction significantly contributed to the upregulation in the expression of BAX as well as to the downregulation in the expression of BCL2 in the treated breast cancer cell (33). This evidence confirmed the antiproliferative activity of the S. sahandica extraction against the cancer cells.
Our study faced a few limitations. First, the effects of the S. sahandica extraction on the cell cycle, autophagic processes, and signaling pathways involved in cancer initiation and progression were not investigated in our study. Second, the effect of S. sahandica extraction on other HCC cell lines was not examined; therefore, it was recommended that this effect should be explored.
5.1. Conclusions
In sum, the S. sahandica extraction exhibited a favorable anti-proliferative activity against the HCC cell line HepG2. It was suggested that the anti-proliferative activity of the S. sahandica extraction may have been due to the induction of apoptosis in the HepG2 cell line. However, LDH nanosheets (Zn3Al) were found to decrease the anti-proliferative activity of the S. sahandica extraction. Therefore, it was recommended that further studies should be carried out in order to investigate the underlying mechanisms of the S. sahandica extraction activity.