1. Background
Rapid advances in computing technology have greatly accelerated computer-aided drug development (CAD). Recently, pharmaceutical companies have turned their attention to traditional Chinese medicine (TCM) to discover new ligands. The Taiwan TCM database is currently the largest non-commercial database in the world based on information collected from Chinese medical texts and scientific publications. This web-based database contains more than 20,000 compounds, 20,000 pure compounds in a two-dimensional format, and 3D available for download for molecular simulation and virtual screening. It is also possible to investigate and study the drug by using a specific search (molecular properties, raw materials, TCM elements, and TCM classification) according to the intended operation (1, 2). The investment of the Chinese central government is very significant in this field. The total budget allocated to TCM research in 2010 was about 4.9 billion yuan (770.5 million US dollars). In 2010, according to the State Administration of Traditional Chinese Medicine, with an increase of about 53%, its use was for research on TCM. As of May 2011, China has signed 91 TCM cooperation agreements with more than 70 countries, aiming to promote and better recognize TCM worldwide (3).
In the last decade, after the first systematic review of the clinical study of TCM in 2002, more clinical trials have been published internationally, and the results have shown that was increased in the number of systematic reviews (SR) and meta-analyses on TCM clinical trials (3). This ratio peaked in 2004, but has gradually decreased since 2005, possibly due to the need for more rigorous clinical trials. However, it seems that the level and quality of published articles have increased (4). Considering the important topic of inflammation, which is mentioned below, virtual screening based on the structure of medicinal plants in Iran is of key importance in order to identify plants that have effective substances against inflammation (3).
Inflammatory disease, especially in the acute stage is detected through increased blood flow and increased vascular permeability, along with the accumulation of fluid, leukocytes, and inflammatory mediators such as cytokines. Meanwhile, lymphoid cells, inflammatory cells, and hematopoietic cells participate in forming an effective immune response (5, 6).
Inflammatory bowel disease (IBD) refers to a group of chronic inflammatory disorders of unknown origin that involve the digestive system. The symptoms of this disease depend on the part involved in the digestive tract. Ulcerative colitis is characterized by inflammation of the innermost mucosal layer of the intestine, while Crohn’s disease occurs with inflammation of all layers of the intestine. These two diseases are debilitating diseases of the digestive system with similar clinical, pathological, and epidemiological characteristics. This disorder is divided into two major conditions, including ulcerative colitis and Crohn’s disease. Recent studies show that interleukin-22, which is a member of the interleukin-10 cytokine family, is involved in inflammatory processes during the course of the disease (7).
Flavonoids help regulate cellular activity and fight free radicals that cause oxidative stress in the body. Simply put, they help the body function more effectively while protecting it from toxins and stressors. Flavonoids are also powerful antioxidant agents. Antioxidants help the body fight against potentially harmful molecules that can be introduced into the body. Inflammation is one of the immune responses of the human body. Allergens, germs, toxins, and other irritants can cause inflammation that leads to uncomfortable symptoms. Flavonoids may help the human body suppress this inflammatory response to reduce these symptoms (8, 9).
Inflammatory bowel diseases (Crohn’s disease) are currently common causes of gastrointestinal disorders in developed countries. A recent study reported a prevalence of IBD of about one patient per 200 people in northern European populations (10). Although death due to IBD is not common now, the disease continues to cause disability and morbidity, especially in young adults. Crohn’s disease, in terms of etiology, is still a challenge. Some use it to protect against diseases, as the immune system attacks its own cells. However, evidence of viral infections, which is the origin of this disease, has been reported (11).
The activation of pattern recognition receptors, due to encountering an intestinal infection, causes the initiation of inflammatory responses and the release of inter-inflammatory cytokines, which increases intestinal diseases (12, 13). Since the treatment of cancer with industrial and chemical drugs is associated with drug resistance and side effects, many studies have focused on the use of medicinal plants and herbal medicines due to the multiple beneficial effects of natural products and less harmful effects compared to conventional chemical treatments.
Lycopene (a carotenoid) has shown its anticancer effect on HT-29 cell lines through inhibition of Akt phosphorylation (14, 15). The cytotoxic effects of a number of plant extracts were investigated on AGS, KYSE-30, HT-29, and L-929 cancer cell lines as a healthy control cell, and the MTT assay showed that Cichorium intybus, Urtica dioica, and especially Arctium lappa had the strongest cytotoxic effects (16). Rosemary extract (Rosmarinus officinalis L.) has a high anti-inflammatory activity, which indicates a higher amount of carnosic acid and carnosol in rosemary (17).
Flavonoids are powerful antioxidants with anti-inflammatory, antibacterial, antiviral, and immune system benefits. Diets rich in flavonoid-containing foods are sometimes associated with cancer, neurodegeneration, and the prevention of cardiovascular disease. Population studies have shown that the consumption of flavonoids is inversely related to mortality from cardiovascular diseases. Flavonoids have been reported to have a beneficial effect on atherosclerosis-related parameters, including lipoprotein oxidation, blood platelet aggregation, and vascular reactivity. Antioxidant, anti-thrombotic, anti-inflammatory, and lipid-lowering properties play an important role in reducing cardiovascular mortality observed with higher consumption of flavonoids. Therefore, recently, flavonoids have been the focus of many current nutritional and therapeutic interests (18, 19).
2. Objectives
Considering the importance of inflammatory bowel diseases, in this research, we aim to identify some main inflammatory receptors in these diseases using NGS data analysis and then introduce inhibitors with the virtual screaming technique in these active plant compounds available in TCM and also the effectiveness of these compounds will be evaluated at in vitro level.
3. Methods
3.1. Place and Time of the Test
This research was performed in January 2018 at University of Zabol. The bioinformatics part of the relevant thesis was done by an indirect connection to a very powerful server through one of the computers of the Zanjan University Data Center, and the main practical part was done at the Biotechnology Research Institute of University of Zabol. All parts of the project, including cell cultures, preparation of materials, supplementary tests, and also real-time PCR (RT-PCR) tests of QIAGEN’s Rotor-Gene 6000 model, were carried out at University of Zabol, Agricultural Biotechnology Research Institute.
3.2. Population Study
The population study included 200 human transcriptomes and about 60 thousand compounds related to medicinal plants (rosemary and nettle). In vitro experiments were conducted with at least three experimental and three technical replicates. In this study, the anticancer and immunomodulatory effects of the aqueous extracts of rosemary and nettle medicinal plants, as a promising source of cancer treatment, were investigated against the AGS cell line derived from gastric cancer (AGS-Cell-DGC). Also, IC50 of the extracts was determined by MTT assay. Flow cytometry and RT-PCR methods were performed.
3.3. Cell Culture, RNA Extraction and Primer Design
First, using the SRA Fastq/SRA Toolkit package, about 200 samples of esophagus, stomach, small intestine and large intestine cancers in four separate categories (50 samples from each disease) compared with 200 healthy samples of these tissues as controls were taken. The data are related to the transcriptome of the samples themselves, and the standard cell lines of these four tissues were not included in the data. Data will be used, which is part of the biopsy of the tissue itself, and transcriptome analysis was performed using several different software. Then the data quality was evaluated with FastQC software (20).
Low-quality reads were also removed. Adapters and sequences from the company that performed their sequencing were identified and removed by this software. Removal was done using Top Hat, Bow Tie, Genius R11 software. The transcriptome of the human genome was downloaded from the Ensembl database. Then the Map Read to Reference test was performed to normalize and calculate the difference in gene expression, the RPKM method was used (21) and the following two analyzes were performed. First, the transcriptome pattern of these data and cancer tissues, compared to the control group, was obtained for all their genes, especially the genes of the innate immune system and pattern recognition receptors, which we mostly focused on receptors TLR1 to TLR10. Second, we focused on the differentiation of these receptors and genes that are in the signaling pathway of these receptors, and the transcriptome pattern was determined using Map Read to Reference statistical tests and RPKM method with CLC Genomics Workbench version 20 software.
Finally, the results of these four different diseases were compared, and among them, a receptor that showed inflammation among all of them was identified, and in the next step, several targets were selected from among them (one or two). The next step is virtual screening, in which more than 50,000 medicinal compounds from the TCM database were implemented (22). Then, preliminary preparation was done in software such as ICM pro, Schrodinger, and CLC Drug Discovery. All possible spatial structures were drawn for them, and molecular docking was done eventually. Molecular docking on the molecules, which were selected in the transcriptome analysis stage, and the final goal is to select from among them, compounds of medicinal plants that have the antagonistic ability for these receptors. Docking and virtual screening (23) on 220,000 compounds were performed using Schrodinger software of the MOE package. In the next step, scoring was done. Compounds that had antagonistic abilities were identified. Medicinal plants that contain these substances were selected from the list that was obtained, and among them, there were Iranian medicinal plants. Finally, medicinal plants of rosemary and nettle were selected for this research (24).
To validate the results of bioinformatics studies, the assessment of inflammatory characteristics in the cell culture medium in vitro was performed. Peripheral blood mononuclear cells were isolated using Ficoll-Hypaque gradient method (25). In an experimental design, it was challenged for two hours with substances whose agonistic or antagonistic ability was confirmed in bioinformatics studies. The cells were harvested and RNA extraction and cDNA were synthesized immediately. Finally, qRT-PCR test was performed using ROTORGEN 6,000 device to check the expression of target genes and Pfaffl method was used to measure gene expression. Primers were designed with Allele ID and CLC main workbench (Table 1). The specificity of primers was done using NCBI database. Primers that connected to off-target regions in all their parts, even in the 5’-end with a number of mismatches, were removed.
| Gene Name and Motif | Temperature (°C) |
|---|---|
| Gene Type: TLR4 Activation | |
| TNF-α | |
| F; 5’-CTCTTCTCCTTCCTGATC-3’ | 51.6 |
| R; 5’-CTTGAGGGTTTGCTACAA-3’ | 53.9 |
| IL-1β | |
| F; 5’-CTTTGAAGAAGAACCTATCT-3’ | 47 |
| R; 5’-CACTTGTTGCTCCATATC-3’ | 52.3 |
| Gene Type: Reference | |
| GAPDH | |
| F; 5’-GGTCAGATCCACAACGGACA-3’ | 58 |
| R; 5’-CACTGCCACCCAGAAGACTG-3’ | 56 |
The Information on All Primers Used in Gene Expression Experiments
3.4. Software Used in Research
Using the maestro module of the Schrödinger software version 10-2, TCM library preparation (http://tcm.cmu.edu.tw/) and receptor preparation, glide preparation, and initial screening were done. Then Molecular docking and virtual screening were performed by ICM software version 3.8-3 ICM software, and 2014.0901 MOE version MOE software(http://chemcomp.com) and workbench version 3 CLC drug discovery software (http://qiagenbioinformatics.com). The classification and evaluation of the obtained scores were done by Microsoft Office Excel 2016. ADME properties and toxicity were calculated using the Catalyst module in Biovia Discovery studio 2016 software, and additional compounds were removed (Table 2).
| Software Name | Link | Application | Reference |
|---|---|---|---|
| CLC Genomics Workbench | http://www.schrodinger.com | Integrated high-throughput data analysis, SNP detection in whole genomes of any size, detection of structural changes in whole genomes of any size. | (26) |
| ICM | http://biotech.bmi.ac.cn/icm/ | It allows for easy configuration and online processing of complex parameters, | (27) |
| MOE software | http://chemcomp.com | Structure-based design, pharmacophore discovery, medicinal chemistry applications, biological applications, protein and antibody modeling, molecular modeling and simulation. | (28) |
| Biovia Discovery studio | http: //Biovia Discovery studio | Provides a series of graphical plots of the data. | (29) |
| FASTQ | http: //www.bioinformatics.babraham.ac.uk/projects/fastqc | A quality control tool for high-throughput sequencing data, data import via FastQ files, BAM, or SAM format is possible. | (30) |
The Name, Link, and Application of All Software Used in This Research
4. Results
4.1. Melting and Proliferation Curve
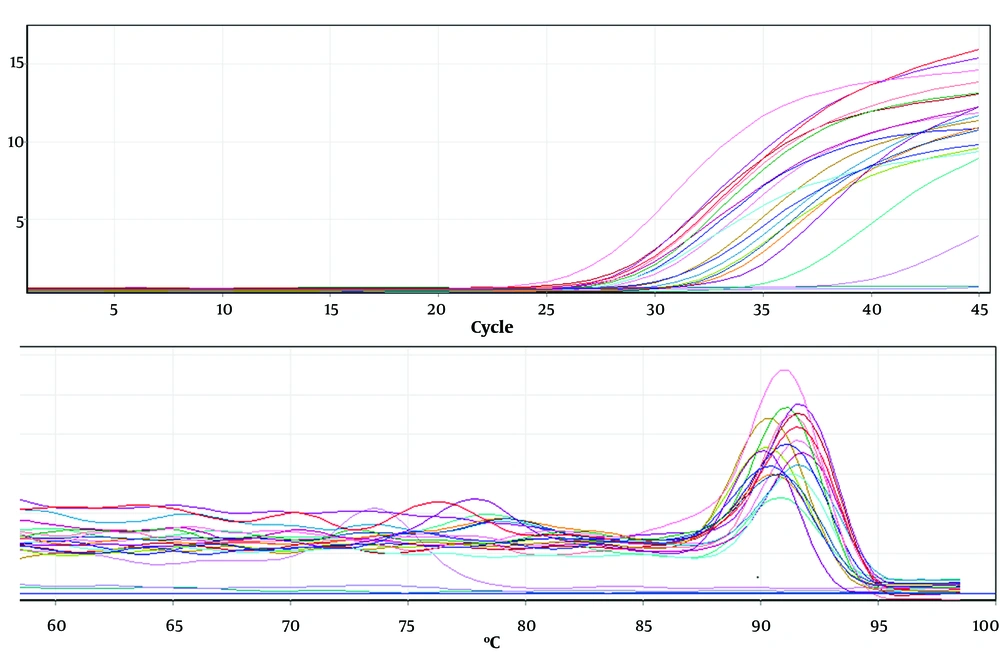
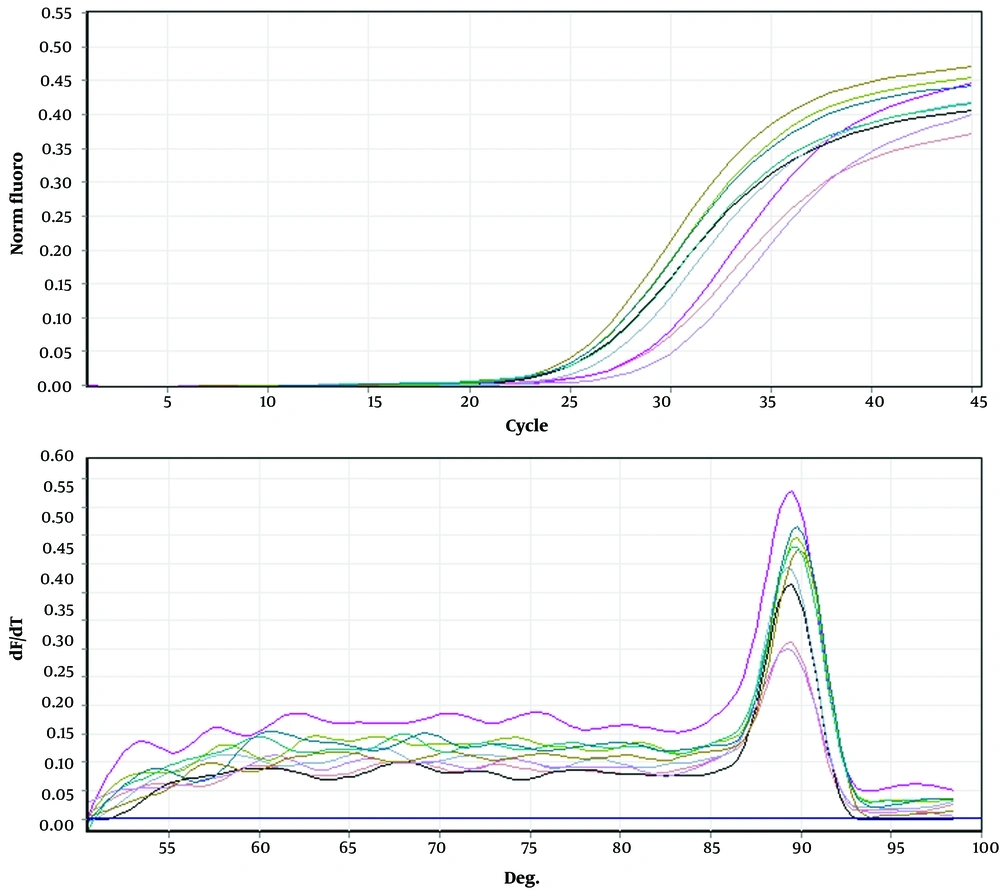
Melting and proliferation curve, the effect of rosemary and nettle extracts on the main gene, and the reference gene of AGS-Cell-DGC were carried out in triplicate. Proliferation curve and melting curve were evaluated for the main gene (NF-κB) (Figure 1) and control gene (GAPDH) (Figure 2) in triplicate in AGS-Cell-DGC, respectively.
4.2. The Results of MTT Test on the Survival Percentage of AGS-Cell-DGC
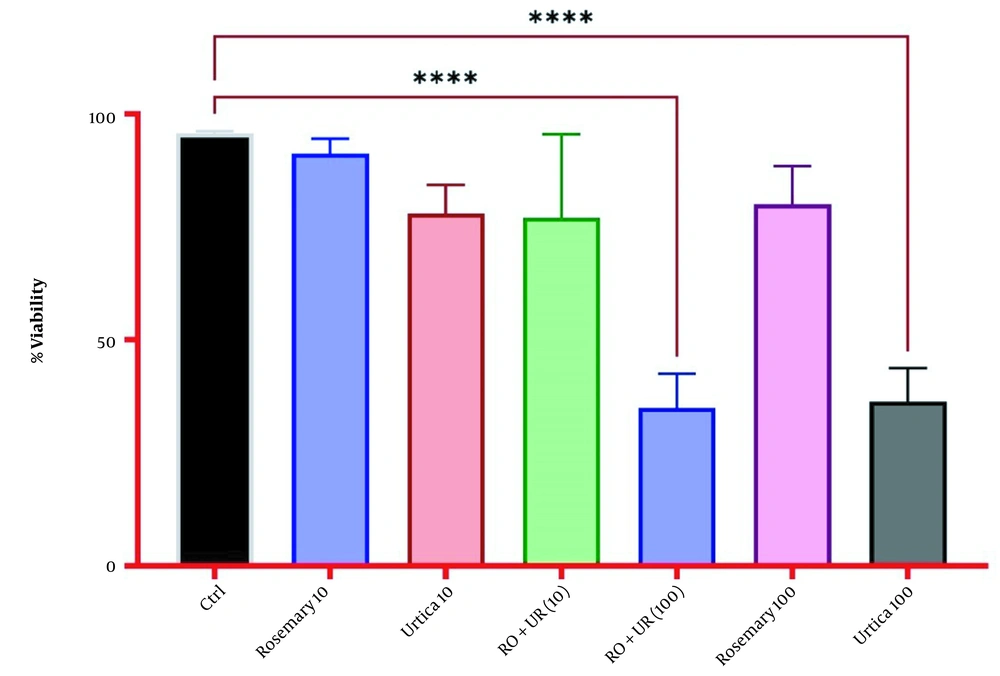
The results of the MTT test for the effect of rosemary and nettle extracts separately and simultaneously on the survival percentage of AGS-Cell-DGC showed that the highest amount of viable cells in the control and the lowest amount in the simultaneous combination of rosemary and nettle extracts and then nettle (100%) was observed (Figure 3).
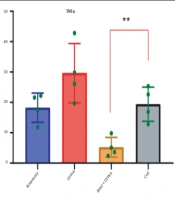
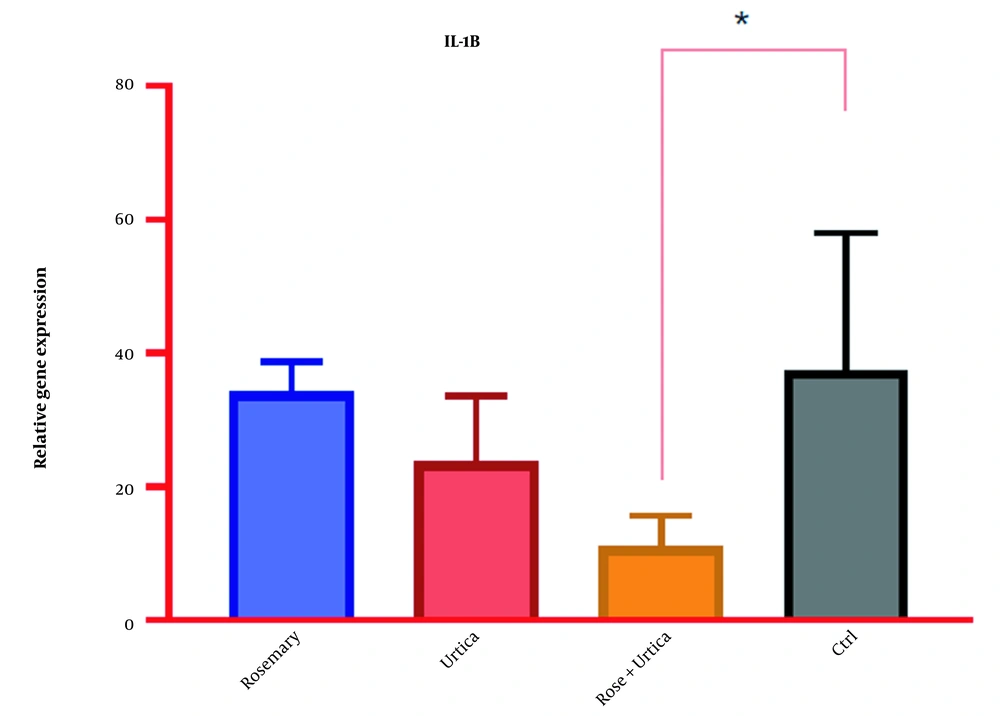
4.3. The Effect of Rosemary and Nettle Extract on Interleukin 1-Beta Gene Expression in AGS-Cell-DGC
Rosemary and nettle extracts, alone and in combination, have provided different results on interleukin 1-beta (IL-1β) gene expression in AGS-Cell-DGC (Table 3). The highest IL-1β gene expression was in the control, and the lowest expression was in the combination of nettle and rosemary extracts (Table 4 and Figure 4).
| ANOVA | SS | DF | MS | F (DFn, DFd) | P-Value |
|---|---|---|---|---|---|
| Treatment (between columns) | 1705 | 3 | 568.5 | F (3, 12) = 4.072 | 0.0329 |
| Residual (within columns) | 1675 | 12 | 139.6 | ||
| Total | 3381 | 15 |
Analysis of Variance of the Effect of Rosemary and Nettle Extract on Interleukin 1-Beta Gene Expression in AGS Cell Line Derived from Gastric Cancer
| Tukey’s Multiple Comparisons Test | Mean Diff. | 95.00% CI of Diff. |
|---|---|---|
| Rosemary vs. urtica | 10.38 | -14.43 to 35.18 a |
| Rosemary vs. rose + urtica | 23.13 | -1.681 to 47.93 a |
| Rosemary vs. ctrl | -3.35 | -28.16 to 21.46 a |
| Urtica vs. rose + urtica | 12.75 | -12.06 to 37.56 a |
| Urtica vs. ctrl | -13.73 | -38.53 to 11.08 a |
| Rose + urtica vs. ctrl | -26.48 | -51.28 to -1.669 b |
Effect of Rosemary and Nettle Extract on Interleukin 1-Beta Gene Expression in AGS Cell Line Derived from Gastric Cancer
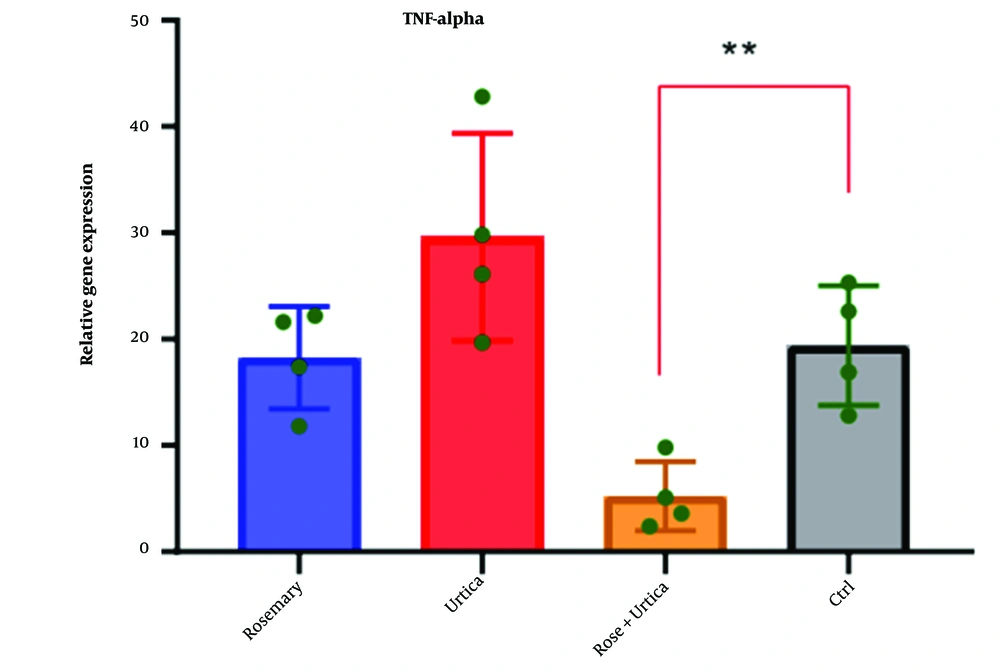
4.4. Investigation of Rosemary and Nettle Extract on Tumor Necrosis Factor-Alpha Gene Expression in AGS-Cell-DGC
Rosemary and nettle extracts, alone and in combination, provided different results on tumor necrosis factor-alpha (TNF-α) gene expression in AGS-Cell-DGC (Table 5). The highest expression of TNF-α gene was in the control and the lowest expression was in the simultaneous combination of nettle and rosemary extract (Figure 5).
| ANOVA | SS | DF | MS | F (DFn, DFd) | P-Value |
|---|---|---|---|---|---|
| Treatment (between columns) | 2370 | 3 | 790.1 | F (3, 12) = 5.775 | 0.0111 |
| Residual (within columns) | 1642 | 12 | 136.8 | ||
| Total | 4012 | 15 |
Analysis of Variance of the Effect of Rosemary and Nettle Extract on Tumor Necrosis Factor-Alpha Gene Expression in AGS Cell Line Derived From Gastric Cancer
5. Discussion
Despite the progress in recent years, as well as the emergence of advanced techniques for cancer diagnosis and treatment, according to the World Health Organization (WHO), cancer is still the second leading cause of death worldwide. Besides, among different types of human cancers, gastrointestinal cancers (esophagus, stomach, and colorectal) are the most common cancers among all the people of the world (31). Currently, various methods such as chemotherapy, radiation, traditional treatments, etc., have been reported to treat cancer, but due to the low specificity of drugs against a specific type of cancer and the resistance of tumor cells to drugs, the effects of these methods and drugs are limited (32). The most basic mode in cancer treatment is to destroy tumor cells without harming the normal cells of the human body. To achieve this ideal condition, it is essential to find new drugs to control all types of cancers.
Today, medicinal plants, as a promising source for cancer treatment, have several beneficial effects on tumor cells with little or no toxicity for patients. Herbal medicines with their active compounds, such as polyphenols, flavonoids, triterpenoids, acetylene, and sulforaphane, can improve DNA damage and stimulate anti-tumor enzymes, anti-inflammatory effects, immune enhancement, and cell adhesion modulation (33, 34). Plant phytochemicals are the first line of treatment for all types of human cancers in traditional medicine. Some studies have shown that medicinal plants and their isolated compounds suppress the expression of certain genes and specific biosynthetic pathways involved in cancer development (35-37). Anticancer activity of ethanol extracts of roots and aerial parts of nine ethnopharmacological medicinal plants of Golestan province (Iran), in terms of their cytotoxicity against L-929, AGS, HT-29, and esophageal adenocarcinoma cells, through promoting apoptosis and immunomodulatory effects. The results showed that out of nine screened plant extracts, A. lappa root (ALR) showed the strongest cytotoxicity (35). In the current research, it was also found that rosemary and nettle extracts, in a combined manner, reduced the expression of genes involved in the production and proliferation of cancer cells.
Among TLRs (pathogen-related molecular receptors), TLR4 (innate immune response mediator in cancer treatment) has been the most extensively studied TLR (38). ERK1/2 and AKT are the main downstream pathways involved in cancer cells. Regulation of the expression of TLR4, AKT, and ERK1/2 in cancer cell lines in response to the treatments of plant extracts has been reported (31). Lycopene (a carotenoid) has shown its anticancer effect on HT-29 through the inhibition of Akt phosphorylation (14, 15, 39). In the present research, it was also found that rosemary and nettle extracts had a positive effect on the reduction of TLR4 gene expression, which was similar to the presented research.
The anti-inflammatory activity of rosemary (R. officinalis L.) extracts was investigated in laboratory conditions, and the secretion and expression of TNF-α, IL-1β, IL-6, and IL-10 genes, as well as the expression of COX-2 gene, were investigated, and it was concluded that rosemary extract has a high anti-inflammatory activity, which indicates a higher amount of carnosic acid and carnosol in rosemary. By comparing the activity of the extract with the standard materials, the anti-inflammatory activity of carnosic acid and carnosol standards was not as high as that of rosemary. As an anti-inflammatory compound, a synergistic interaction with other compounds may play an important role in enhancing its activity. The results of the presented research have provided the basis for a possible increase in the use of supercritical extracts of rosemary in food formulations to reduce or prevent inflammatory diseases (17). In the present research, in most cases, the greatest effect of rosemary on the expression of the investigated genes (such as IL-1β) was synergist with nettle extract, and it had very little effect alone.
The anti-inflammatory activity of rosemary extract enriched with carnosic acid and carnosol was investigated. The results showed that carnosic acid and carnosol significantly reduced the expression of IL-1β and TNF-α and completely inhibited the expression of COX-2. The inhibitory effects of carnosic acid on LPS-induced NO and TNF-α production have been related to the suppression of iNOS and COX-2 expression due to the inhibitory effect on NF-κB induced signaling. Carnosol reduced LPS-induced iNOS mRNA and NF-κB inhibitory kinase activity in mouse macrophage RAW264.7 cell line. However, the commercial use of carnosic acid and carnosol has been limited due to the cost of purification methods. In addition, some extracts or fractions of rosemary have been reported to have similar or superior anti-inflammatory activity than carnosic acid or carnosol alone (17). The results of the inhibitory effect of rosemary extract along with nettle extract on the expression of genes involved in cancer in the current research are consistent with the previous studies.
Although conflicting reports have indicated that flavonoids have beneficial effects on humans and are associated with the prevention of arteriosclerosis, high blood pressure, etc. also, the results of the current research and the review of sources confirmed the use of rosemary and nettle plants, which have various types of phenolic and phytochemical substances and have been used in traditional medicine for thousands of years in the treatment of many diseases. It should be noted that flavonoids in a diet are related to reducing the risk of chronic diseases, all of them are pre-clinical evidence, and to further strengthen this evidence, more confirmation and investigations are needed to fully determine their validity (40, 41). In general, it will still be very difficult to conduct such clinical trials accurately and determine the structure. Also, it is not yet clear for what type of disease, how many phenolic and phytochemical substances and for how long it should be consumed.
5.1. Conclusions
Rosemary and nettle extracts alone and in combination have provided different results on the expression of genes involved in the AGS-Cell-DGC The highest amount of live cells was observed in the control and the lowest amount was observed in the simultaneous combination of rosemary and nettle extract and then nettle (100%) alone.