1. Background
Zinc, an essential element for human survival, is one of the most important structural components of insulin, zinc finger proteins, and superoxide dismutase enzyme (1, 2). This micronutrient is an indispensable catalytic element, exerting an important role in homeostasis, immune responses, oxidative stress, and aging (3, 4). Several studies have shown that zinc is the fourth most consumed mineral in the world, following iron, aluminum, and copper (3, 4). Since this element is part of the body's antioxidant system, its deficiency leads to an impaired antioxidant defense system and ensuing oxidative stress (5). About a quarter of Iranian households have zinc deficiency (6). Hence, it can play a pivotal role in cellular health and homeostasis (7-9). Several studies have shown zinc's anti-cancerous effects, including prevention and treatment of hair loss, enhancement of immune system activity, wound healing, and acne improvement (1, 2, 10). Previous studies have also reported that zinc can prevent molecular degeneration and, therefore, is very useful in treating arthritic rheumatoid and Wilson disease (11-14). In addition, some studies have highlighted the need for a large amount of zinc in treating enteropathic acrodermatitis, alcoholism, diabetes mellitus, kidney disease, and inflammatory bowel disease (3, 4, 15). Zinc deficiency is considered a very serious health problem since it is the fifth most important cause of disease, leading to morbidity and mortality (11-13). Many studies have shown that zinc deficiency in humans causes postponed growth, skin lesions, and sexual dysfunction in adolescents (16-19). The need for zinc in childhood is crucial because of rapid growth, the main characteristic of this age span. Zinc deficiency causes more than 800,000 annual deaths in children around the world. Zinc deficiency in humans is caused by reduced dietary intake, poor absorption, increased loss, or increased body system consumption (11, 14). According to the World Health Organization (WHO), the prevalence of zinc deficiency has reached 31% in the world (14, 20, 21).
Vitamin B12, or cobalamin with the chemical formula C63H88CoN14O14P, is a water-soluble vitamin obtained through fish, meat, dairy foods, cereals, and supplements (22, 23). The amount of vit B12 in the body depends on age, gender, and physical condition. But in general, the normal amount of vitamin B12 in the body can be between 160 and 970, and its critical amount is more than 1000 and less than 160. Cobalamin is one of the most important vitamins, playing a crucial role in cell production, blood cell proliferation, nucleic gene function, and the performance of the nervous system through the production of myelin (24, 25). Cell proliferation occurs in the nervous tissue, digestive tract, and bone marrow due to the presence of vitamin B12 (7, 26). Studies have determined that impaired intrinsic factor synthesis by gastric tissue is the most common cause of vitamin B12 deficiency (27, 28). Various studies have demonstrated that vitamin B12 deficiency can lead to anemia, weakness, depression, sore mouth and tongue, mental disorders, memory loss, weight loss, gait impairment, and improper breathing (29-32). Hence, maintaining a normal level of vitamin B12 in the body through food intake can help to get sufficient amounts; without the need for supplements (29-32).
Many studies have been conducted to determine the properties of zinc and vitamin B12 and their effects on health and well-being (11, 21, 33-36). However, the exact relationship between the two essential factors has remained unknown.
2. Objectives
This study aimed to investigate the correlation between zinc deficiency and vitamin B12 levels in subjects of different ages.
3. Methods
3.1. Research Subjects
A total of 200 male and female patients, who were admitted to the Pasteur Medical Diagnostic Laboratory in Ahvaz in 2019, were selected for investigation. Inclusion criteria required individuals to have no metabolic diseases, autoimmune diseases, malignancy, immunodeficiency, any viral diseases such as hepatitis C virus (HCV), hepatitis B virus (HBV), and human immunodeficiency virus (HIV), as well as hypersensitivity and allergic diseases. Individuals with chronic gastritis and atrophic, prenatal anemia, and stomach removal were excluded from the study.
3.2. Sample Collection and Preparation
Venous blood samples were obtained with consent from patients after overnight fasting and collected into test tubes without anticoagulants. The serum was isolated and then stored at -70°C until the test was conducted. After sample collection, demographic information was acquired through recording and completing the checklist for each sample.
Serum zinc level was determined and measured using Greiner Bio-One kit (North America, Inc) and atomic absorption spectrophotometry (Perkin Elmer Zeeman 3030, CT, USA), respectively. Furthermore, the serum level of vitamin B12 was determined by electro-chemiluminescence, using Elecsys and Cobas analyzer kit (Roche, USA) and Roche Cobas e411 analyzer (Germany). Samples were also divided into three groups, including low serum zinc level (less than 66 µg/mL), normal serum zinc level (between 66 and 110 µg/mL), and high serum zinc level (greater than 110 µg/mL) for determination of the correlation between zinc deficiency and serum vitamin B12 level. Finally, the amount of serum vitamin B12 was compared in the aforementioned groups.
3.3. Statistical Analysis
We used SPSS version 16 software for data analysis. Descriptive statistics were used to report demographic indices, including frequency distribution table, central and dispersion indices, and percentages. Inferential statistical methods, including the Kolmogorov-Smirnov test, Levene's test for equality of variances, independent t-test, and Mann-Whitney tests, were also considered to make a comparison among study groups. Data were reported as mean ± SD, and P values lower than 0.05 were considered significant.
4. Results
4.1. Demographic Indices
In this study, 200 samples constituted 92 (46%) male and 108 (54%) female subjects investigated. The mean ± standard deviation (SD) of age in the studied samples was 39.94 ± 19.22 years (Table 1).
4.2. Zinc and Vitamin B12 Serum Levels
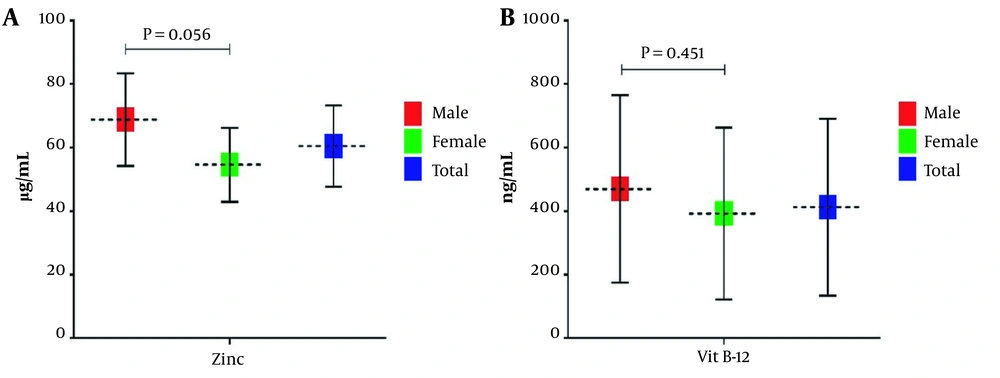
To assess serum zinc level, we used the atomic absorption spectrophotometry method. Vitamin B12 serum level was also determined using an electro-chemiluminescence assay. Evaluation of zinc and vitamin B12 levels revealed that all samples' mean serum zinc and vitamin B12 levels were 60.43 ± 12.81 µg/mL and 412.36 ± 278.56 ng/mL, respectively. Specific evaluation for zinc serum level showed that the mean serum zinc level is 65.78 ± 14.60 in men and 54.58 ± 11.59 µg/mL in women. Furthermore, the mean serum level of vitamin B12 in male and female samples were 469.63 ± 295.31 and 392.24 ± 270.59 ng/mL, respectively. As apparent from Figure 1A and B, compared to women, the mean serum level of zinc and vitamin B12 was higher in men. However, statistical analysis showed no statistically significant difference between the two genders (P > 0.05).
4.3. Age Correlation with Serum Levels of Zinc and Vitamin B12
Analyzing the correlation between the age of study subjects with zinc and vitamin B12 serum levels showed that with increasing one year of age, zinc serum levels decreased for 0.13 units (P = 0.008, β = -0.13, R square = 0.03, 95% CI: -0.23 - 0.036) and vitamin B12 serum levels increased for 0.19 units (P = 0.790, β = 0.19, R square < 0.001, 95% CI: -1.84 - 2.48) (Table 2).
4.4. Cluster Analysis Based on Zinc Serum Level
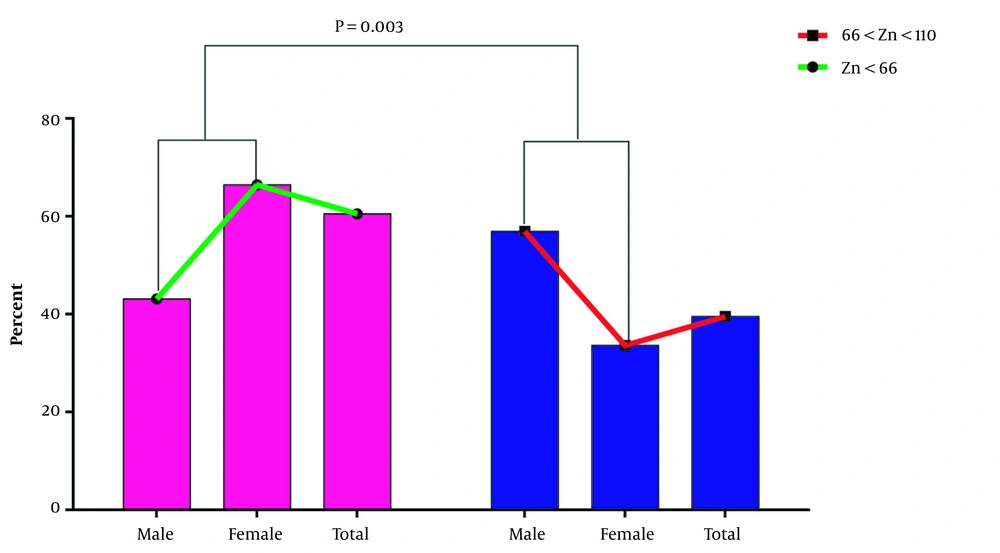
Analysis of the serum zinc level in two groups rendered this result: of the 200 studied samples, 121 (60.5%) had lower serum zinc levels (group 1), and 79 samples (39.5%) had normal serum zinc levels (group 2). Further evaluation of the sample showed no sample with higher serum zinc levels. The results indicated that 56.9% of men and 33.6% of women had normal serum zinc levels. Moreover, a statistically significant difference was found between the level and gender (P = 0.003). According to the data analysis, the mean serum level of zinc in group 1 and group 2 were 52.66 ± 8.83 and 72.32 ± 7.85 µg/mL, respectively. The data in Figure 2 shows that the serum level of zinc in group 1 is lower than in group 2. However, the observed discrepancy was insignificant (P = 0.085).
4.5. Zinc Deficiency Relationship with Serum Levels of Vitamin B12
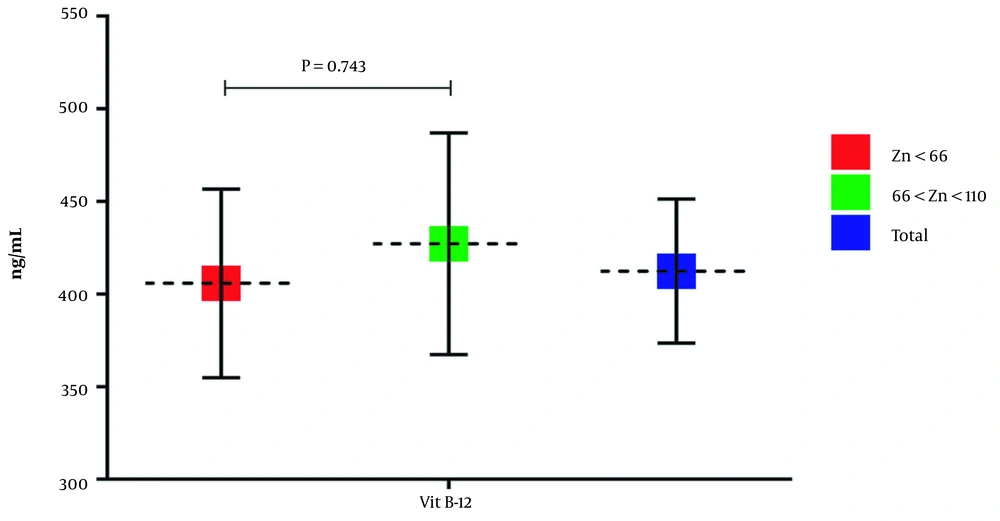
The results showed that the serum vitamin B12 level of group 1 and group 2 is 405.75 ± 282.7 ng/mL and 427.13 ± 267.01 ng/mL, respectively. According to Figure 3, the serum vitamin B12 level of group 1 is lower than group 2, but the observed difference was not considered statistically significant (P = 0.743, mean diff = -21.37, SD = 40.01, 95% CI: -100.28 - 57.53).
5. Discussion
The present study was designed to determine the correlation between zinc deficiency and the status of vitamin B12. In this study, zinc and vitamin B12 were found to have a positive correlation. However, this finding did not show a statistically significant correlation. This finding is in agreement with the findings of Ozuguz et al., which showed no significant relationship between serum zinc and vitamin B12 levels in the studied patients (37).
Results of the current study showed that in comparison to the group with lower zinc levels, the group with normal levels of zinc had a higher amount of vitamin B12. Nevertheless, the difference between the two groups was not statistically significant. It is difficult to explain this result, but it might be due to the proximity of zinc content between the two groups. Therefore, it can be inferred that with increased levels of serum zinc in the two studied groups, there would be a significant increase in the level of vitamin B12. Moreover, this result may be because vitamin B12 is stored in the liver; hence, if a person has not used this vitamin for a while, there would still be no state of deficiency in the body (29-32). These findings further support the idea of some studies that have suggested a likely interaction between zinc and vitamin B12 and the developmental impacts of both nutrients on each other (21, 31, 37, 38).
In contrast to the results of our study, Betul Ergul et al., the study showed that in the zinc-deficient group, vitamin B12 level was significantly higher than in the normal group (19). It can be inferred that these discrepancies might be due to individual differences and variant dieting patterns among different populations.
It is somewhat surprising that in assessing the relationship between age and serum levels of zinc and vitamin B12 in this study, results showed that serum zinc levels decreased and levels of vitamin B12 increased in an age-dependent manner. It is also important to notice that, contrary to the case of vitamin B12, these findings were statistically significant for zinc. Our findings seem to be consistence with other research, which found a clinically significant difference in vitamin B12 and zinc content between Helicobacter pylori-infected subjects and healthy ones. However, they declared that the observed relationship wasn't statistically significant (21).
Our study showed that a high proportion of the participants (60.5%) were suffering from zinc deficiency. Also, it was revealed that zinc deficiency is more common in women than men, indicating the possibility that women are at more risk. This finding corroborates the idea of Hashemi et al., who demonstrated that the serum zinc level in women is lower than in men. They also reported that 60.2% of men and 77.5% of women have a lower-than-normal serum zinc level. However, they observed no significant relationship between serum zinc concentration and age or BMI (39).
According to the study of Kruis and Phuong Nguyen, zinc deficiency can be the risk of developing in patients with Crohn's disease. They reported that chronic intestinal inflammation could impair vitamin B12 uptake, representing lower serum levels of vitamin B12 in patients (31).
The results of our study showed that the mean vitamin B12 in the studied samples was in the normal range. It seems that this is due to sampling selection from a population of healthy individuals. In future investigations, it might be possible to select samples from different groups, in which the analysis of zinc and vitamin B12 correlation would be more reliable. Therefore, it is recommended for future research to study a wide range of individuals in different populations.
5.1. Conclusions
This study set out to determine the correlation between zinc and vitamin B12 status in a selected population. The current study found that the frequency of samples with zinc deficiency is generally very high. Also, the results show that the serum level of vitamin B12 in people with a serum level lower than the normal level of zinc is lower than that of people with a normal serum level of zinc. Based on these findings, it may be beneficial to repeat this study with a larger sample size and a greater variety of populations in order to obtain reliable results.