1. Background
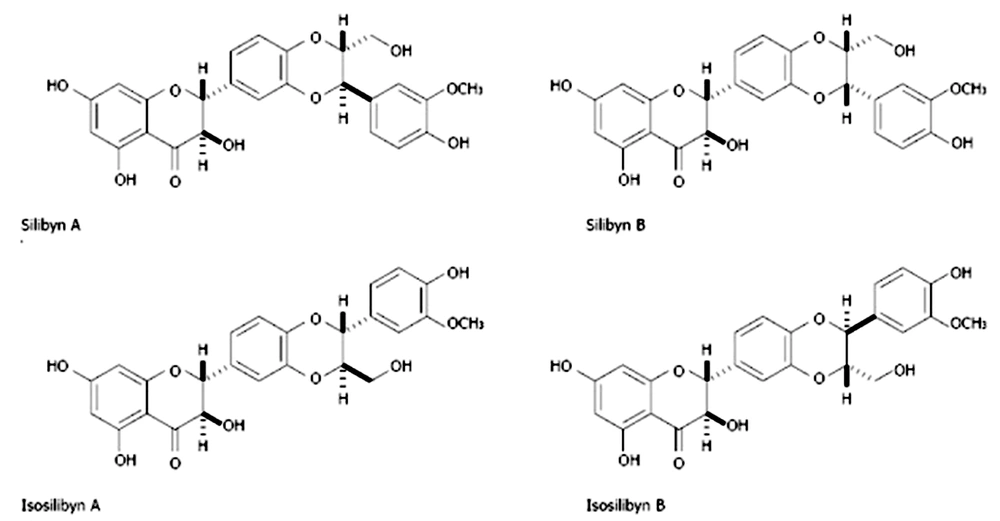
Biodegradable polymers are receiving greater attention today because of the environment's importance. One of these polymers, the linear, hydrophobic, semi-crystalline aliphatic polyester polycaprolactane, is an example. Microorganisms steadily break down polycaprolactane in the environment. Due to its superior physical characteristics, accessibility, and intrinsic biocompatibility, it is frequently employed in tissue engineering. One of the drawbacks of polycaprolactane, which makes it a hydrophobic polymer, is the absence of functional groups on the surface of polymer chains. In various domains of tissue engineering, the use of polycaprolactane as a viable biodegradable material has recently been studied (1). Due to their non-toxicity, biodegradability, biocompatibility, cost-effectiveness, and accessibility, natural polymers have garnered more attention for medical applications than synthetic polymers. Traditional Chinese medicine has extensively used tragacanth and has a long history of doing so. Numerous clinical studies on treating diabetes, cancer, and constipation have used tragacanth alone or in conjunction with other herbs. Tragacanth is a painkiller used in traditional Iranian medicine to heal sore throats and hair loss from greasy skin. Antiviral and antimicrobial effects have been suggested for sterilization in contemporary medicine. Tragacanth is a natural polymer, and since some natural polymers are found in the extracellular matrix's structure, it can be used in tissue engineering since it has strong cell adhesion. Tragacanth has no sensitizing, mutagenic, embryo-defecting, carcinogenic, or toxic effects on humans, and it fosters an environment conducive to cell growth (2). The naturally occurring substance known as silymarin can be found in milk thistle, a derived species of the Silybum marianum plant. At least seven flavonoids, including taxifolin, are present in this plant. The three flavonoids, silybin, silydianin, and silychristin, are the most significant (Figure 1). Between 50 and 70 percent of silymarin extract is silybin (3). Due to its positive effects on treating liver problems, silymarin has been utilized as an alternative supplemental medicine for many years. Silymarin is a member of the asteroid family (Asteraceae or Compositae). This mature plant's big, vivid purple blossoms are covered with thorns. This plant thrives in areas with sufficient sunlight (4).
Patients with damaged livers experience chronic inflammation. As a result, the bioavailability of silymarin's components may be impacted in patients with decompensated cirrhosis, hepatitis C, and nonalcoholic steatohepatitis, which may help to explain why treatment with flavonoids is ineffective in these patients (5, 6). The antiproliferative and cytotoxic potential of these substances against human prostate cancer cell lines was examined. Some malignancies are less common because of silymarin (7). At a low dose (80 mol/L), silymarin boosted the expression of Bcl-2 and decreased active caspase-3 or apoptosis-inducing factor (AIF) in nasopharyngeal cancer cells (NPC-TW01) (8). According to theories, silymarin also protects against genomic damage, boosts protein production in liver cells, and inhibits the action of tumor promoters (9).
2. Methods
2.1. Preparation of Scaffolding
The electrophoretic technique was employed to create the scaffolds used in this study. An electrospinning machine with a rotary collector that is 70 mm thick and 50 mm wide was employed for this project. In this study, the scaffold was made by combining, in a ratio of (2:3), a 0.9 percent weight concentration of silymarin solution with a 7 percent solution of polycaprolactane that was dissolved in a solvent containing acetic and formic acid. Sodium didecyl sulfate (SDS) was added to a solution of 0.7 percent by weight concentration of PCL and mixed with a magnetic stirrer for 20 minutes. To make the solution uniform, SDS was added at a concentration of 1 percent by weight relative to the solvent. The suspension was then ultrasonically sonicated for 20 minutes. To create nanofibers, it was homogenized and fed into an electrospinning apparatus. The nanofiber samples were collected by spinning at 250 rpm for 5 hours, with the sample collection speed being 1 mL per hour. The process is carried out at a voltage of 20 kV, and the distance between the injection needle and the scaffold is 15 cm (10).
2.2. Examining the Porosity of Scaffolds
To measure the scaffold's porosity and the diameter of the fibers, a scanning electron microscope (SEM) was employed. Imaging was done after the samples were coated with gold.
2.3. Investigating the Chemical Structure of Scaffolds
FTIR spectroscopy was employed to identify the chemical composition of the produced scaffold. In order to achieve this, a Japanese-made SHIMADZU spectrometer was used to create an FTIR spectrum with a wavelength range of 400 - 4000 cm-1.
2.4. Cell Culture
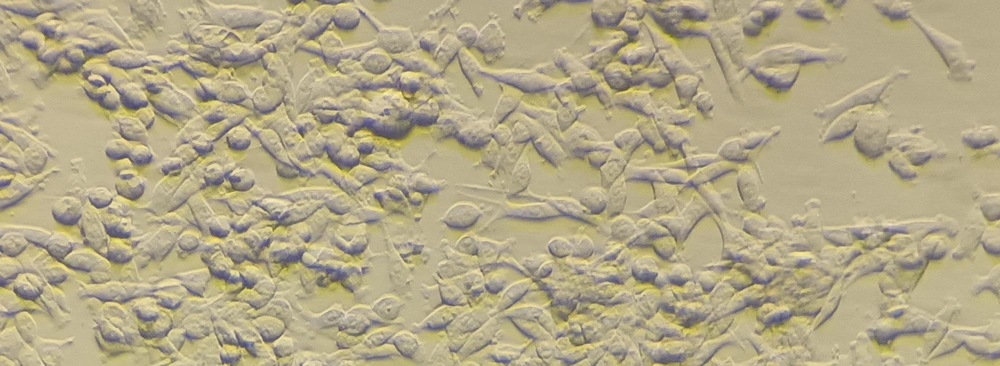
Cells (PC12) for this study were acquired from Iran's Pasteur Institute. The cells were created in RPMI culture media (Gibco, England) with 10% fetal horse serum, 5% fetal bovine serum, and 100% fetal calf serum. Penicillin-streptomycin, a medication produced by Gibco in England, was cultivated in an incubator at 37 degrees and 5 percent CO2 and contained one unit per milliliter. (Figure 2).
2.5. Scaffold Preparation Before Cell Culture
The prepared scaffolds were divided into 1 square cm-sized pieces before being inserted into the wells of the 24-well plate for cell culture. The scaffolds inside the wells were rinsed twice with PBS buffer for 10 minutes. The scaffolds under the hood were exposed to UV light for 20 minutes. On each of the 24-well plate scaffolds, 50,000 PC12 cells were grown after the scaffolds had been sterilized.
2.6. Investigating the Metabolic Activity and Biocompatibility of the Scaffold
Using MTT, it is possible to identify living cells in various biological samples. The materials are mixed with tetrazolium, a yellow chemical or MTT reagent, in this procedure. The mitochondrial enzyme succinate dehydrogenase breaks down tetrazolium to produce insoluble formazan (11). The following steps have been taken in applying this method: A 96-well plate containing ten microliters of the solution (5 percent MTT) was added and incubated for two hours at 37 degrees. The next step involved dissolving cells and colored crystals in 100 microliters of DMSO. An Elisa device was then used to measure the optical absorption rate at 570 nm (the reference wavelength is 620 nm). Cellular longevity and absorption rate are closely connected (12).
3. Results
3.1. Investigating the Nano Scaffold Structure and Its Non-toxicity for PC12 Cells
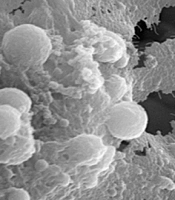
Scanning electron microscopy images of the scaffold surface without cells (Figure 3) and with cells (Figures 4 and 5) were created after the scaffolds had been constructed to assess the structure, porosity, and cell survival on the scaffold (SEM).
The silymarin/tragacanth scaffold and the polycaprolactane and silymarin scaffold after cell culture is depicted in Figures 4 and 5, respectively. It may be said that the silymarin/PCL scaffold and the silymarin/ tragacanth scaffold are suitable for biocompatibility due to the adequate porosity of the scaffold and the presence of cells on the scaffolds. PC12 cells are present.
3.2. Investigation of FTIR Spectroscopy of Silymarin/PCL Scaffold
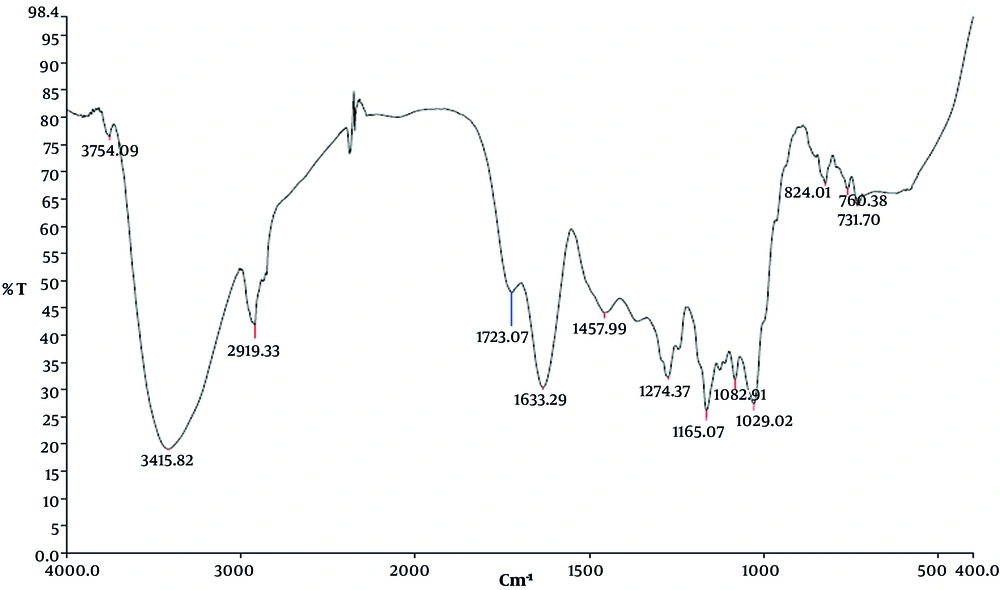
As seen in Figure 6, the absorption spectrum of polycaprolactane typically exhibits three peaks at wave numbers cm-1 1723, 2865, and 2919, which denote the presence of bond vibration and stretching vibration of -C=O bonds. -CH2. Silymarin index peaks at wavelengths cm-1, 1029, 1082, and 1274 show the vibration of -C-O bonds, 1731 shows the vibration of -COOH carboxylic acid, and 1508 cm-1 shows the vibration of aromatic C=C. It is due to the -O-H stretch in the silymarin structure, which denotes that silymarin was properly coated onto the polycaprolactane scaffold (13, 14).
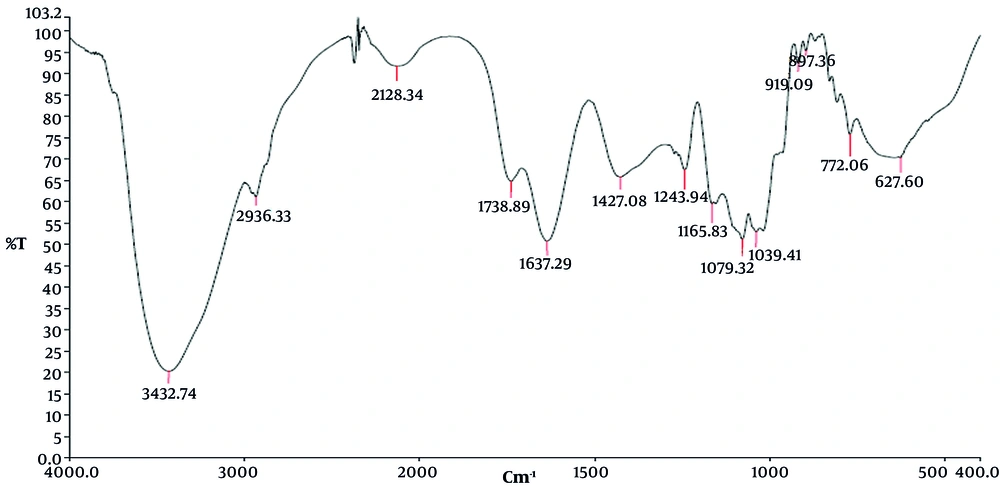
3.3. Investigation of FTIR Spectroscopy of Silymarin/Tragacanth Scaffold
Gum's spectra contained significant absorbance bands at 3432, 2936, 2128, 1738, 1637, 1427, 1243, 1165, 1079, and 1039 cm-1, respectively. The broadband seen at 3432 cm-1 may be attributed to the O-H group stretching vibrations in the gum. The asymmetric and symmetric stretching vibrations of methylene groups, respectively, are shown by the bands at 2936 cm-1. Different gum carbonyl species cause the wide band at 2128 cm-1. Aldehydes, ketones, and carboxylic acid's carbonyl stretching vibrations may be responsible for the sharp peak at 1738 cm-1. The more pronounced band at 1637 cm-1 may be attributed to the distinctively asymmetrical stretch of the carboxylate group. The bands located at 1427 cm-1 give the carboxylate group its symmetrical stretch. The C-O stretching vibrations of polyols, ether, and alcoholic groups were responsible for the peaks at 1243 and 1165, 1079 and 1039 cm-1, and correspondingly (Figure 7).
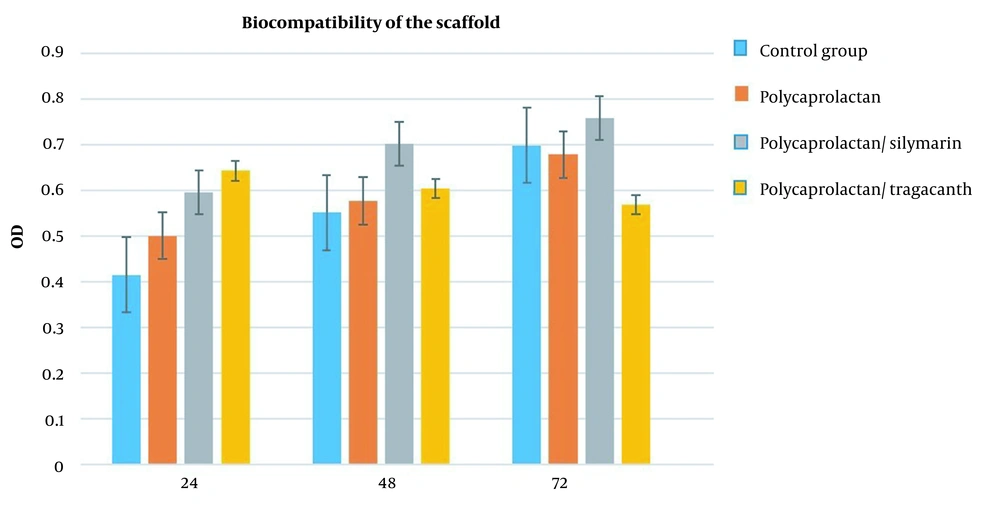
3.4. Biocompatibility of the Scaffold
The MTT test was utilized following the biocompatibility test to validate the vital activity of the cells, development, and multiplication of the cells on the employed scaffold. Three different time frames of 24, 48, and 72 hours were used for the MTT test. The graphs below display the results of the MTT test. The results were shown in a graph over three-time intervals and three repetitions for each sample, and the polycaprolactane/silymarin scaffold considerably outperformed the L-polycaprolactane/ tragacanth scaffold, polycaprolactane, and the control group in terms of cell survival rate (Figure 8).
4. Discussion
Tissue restoration is the goal of tissue engineering, which creates a scaffold out of powerful synthetic materials like biomaterials and biological tools like cells. Cells are arranged in three-dimensional microenvironments and encircled by ECM and other cells in an organism's developed tissue. In this study, the results of the SEM electron microscopy and MTT tests demonstrate that the polycaprolactane/silymarin and polycaprolactane/ tragacanth scaffolds are biocompatible and non-toxic and that after cultivation, the polycaprolactane/silymarin scaffolds significantly outperformed the polycaprolactane/tragacanth scaffolds in terms of cell survival. A cell, that is. The biocompatibility of the polycaprolactane/ tragacanth scaffold and polycaprolactane/silymarin scaffold were also compared, and the results demonstrated that silymarin loading on polycaprolactane scaffold improved the scaffold's biocompatibility. Cell behavior is a culmination of several signaling events that happen as a result of interactions between nearby cells with soluble substances, and it occurs with ECM (15, 16). The ECM is an intricate and regular network of nanofibers, and the nanotopographical environment has an impact on signaling pathways that determine cell phenotypic and fate. As a result, nanofiber scaffolds have gained attention recently (17, 18). The electrolysis method was used in this work to choose polycaprolactane polymer as the scaffold material. This polymer, which is linear and hydrophobic, is frequently employed in tissue engineering because of its outstanding physical qualities, availability, and biocompatibility. It is also thought to be a viable material for use in medicine. Silymarin (milk thistle), a flavonoid with numerous benefits, including an anticancer impact and antioxidant capabilities, was also utilized as a loading agent. Silibinin is the most significant component of silymarin, an extract made up of numerous isomers and accounts for roughly 80% of this extract (19). Tragacanth is a natural polymer, and since some natural polymers are found in the extracellular matrix's structure, it can be used in tissue engineering since it has strong cell adhesion. Tragacanth creates a favorable environment for cell growth in the human body while having no sensitizing, mutagenic, carcinogenic, embryo-defecting, or toxic effects. Myofibroblasts, which can close, are what make tragacanth so successful at healing and regenerating wounds. The active components of tragacanth (tragacanthinand tragacanth) aid in the rapid creation of collagen and the healing of wounds. tragacanth has antibacterial, wound-healing, and healing characteristics in addition to the capacity to regulate the release of drugs from drug-delivery devices. Additionally, tragacanth's antibacterial qualities make it useful for antimicrobial systems and wound dressings. Wicking can cause the fiber diameter during electrospinning to decrease. This organic polysaccharide has produced effective drug encapsulation outcomes as well. tragacanth is a naturally occurring polymer that is produced from various exudations. In addition to modest amounts of protein, starch, and cellulose components, this polymer is a complex mixture of water-soluble and insoluble polysaccharides. Tragacanthic acid, also known as bassorin, makes up between 60 and 70 percent of the weight of tragacanth and is a combination of Ca, Mg, and K salts that are insoluble in water. tragacanth has been utilized in a variety of fields, including medicine and the food industry, as a preservative and softener. In medicine, tragacanth has been used to create ointments to cure wounds in animal models and to prepare mucilage for treating burn burns. Cell adhesion is one of the useful characteristics of scaffolds for use in tissue engineering (20, 21). Scanning electron microscopy micrographs from our investigation revealed that the cells were firmly linked to the nano scaffolds and one another, demonstrating the strength of this attachment. In a study, Mehri et al. examined doses of St. John's wort ethanolic extract on the PC12 cell line at 2.5, 5, 10, 20, 50, and 100 g/mL; none of these concentrations had any harmful effects on this cell line. They were consequently selected as appropriate concentrations for this study. The toxicity produced by acrylamide might be decreased by placing the stated cell line in close proximity to St. John's wort extract for 24 hours at concentrations of 2.5, 5, 10, 20, 50, and 100 g/mL. In PC12 cells, proximity to acrylamide led to an increase in cytotoxicity caused by reactive oxygen species, but proximity to crocin dramatically decreased cytotoxicity by reducing oxidative stress (22). The choice of scaffolding material, in addition to the manufacturing technique, is crucial to the success of skin tissue engineering. Scaffolds have been created using a variety of organic and synthetic materials, including gelatin, PLL, PCL, collagen, silk fibroin, and chitosan. Natural polymers' poor mechanical qualities are their main barrier to use. Hydrolytic degradation of synthetic polymers, like PLLA and PC, eliminates their byproducts through the metabolic pathway. Compared to natural polymers, these polymers offer better mechanical properties and are simpler to produce (23). Sharifi Ferdoey et al. modified the PCL scaffold treated with plasma in order to investigate the proliferation of fibroblast cells. They used a mouse. The results showed that the PCL scaffold modified with plasma, in comparison with the PCL coating scaffold given with gelatin-chitosan, had the ability to support less Cultured cells for appropriate cellular response. They are on the scaffolding (19). In a study by Ranjbar Mohammadi and Bahrami compared to pure PCL, GT addition resulted in a large reduction of fiber diameter and changing fiber morphology. Produced scaffolds from 7% GT and 20% PCL had better morphology, and a composition of 3:1.5 (PCL/GT) with a high amount of GT was selected for further experiments. Human fibroblast and NIH 3T3 fibroblast cells adhered and proliferated well on PCL/GT scaffolds. The hydrophilicity nature of nanofibers, degradation behavior, mechanical strength, good morphology of cells on PCL/GT nanofibers, and cytotoxicity assessing methods showed that these scaffolds are safe and have the potential to be developed as skin scaffolds or wound dressing patches (2). Ranjbar Mohammadi et al. showed that the ratio of (2:2.2:0.8) with more GT in its structure and the mean diameter of 130 ± 19 nm was appropriate. Since the PCL-GT (2:2.2) formulation could not form proper fibers, the PCL-GT (2:1) composition was prepared and used for better comparison of PCL-GT-PVA and PCL-GT specimens in subsequent tests. PCL-GT-PVA nanofibers exhibited tensile strength. And young modulus about 2.7 and 56 MPa, respectively. Mesenchymal stem cells on the scaffolds demonstrated attachment and proliferation of cells (24).
4.1. Conclusions
Overall, the results of the SEM electron microscope and the MTT test demonstrated that the polycaprolactane/silymarin scaffold was much more biocompatible than the polycaprolactane/tragacanth scaffold. In order to apply neural tissue engineering to the repair of neural injuries, it appears that the polycaprolactane/silymarin scaffold can be a promising candidate.
4.2. Research Limitations
The absence of an assessment of scaffolds in animal models is one of the research's drawbacks. Future research should consider using polycaprolactane nano scaffolds containing silymarin to restore nerve injury in rats.