1. Context
Maintaining and developing the quality of life depends on lifestyle, and regular aerobic exercise is one of the factors that improve the quality of life by modifying the lifestyle (1-5).
Although the production of reactive oxygen species (ROS) increases in response to regular physical exercise, the improvement and development of the antioxidant defense system is also created in parallel with this increase and protects the tissues against the damage caused by ROS (2, 3, 6, 7). Recent research shows that ROS has a beneficial role in promoting muscle adaptations to physical exercise (3, 6, 8). Skeletal muscle is active during the performance, and the optimal amount of ROS is essential in creating adaptations in the muscle. Reactive oxygen species can induce several signaling pathways in skeletal muscle, which causes physiological adaptations in this tissue. At the same time, excessive ROS causes impaired contractile function and muscle weakness (9).
But the increase in ROS level and the decrease in gene expression of antioxidant enzymes, which are considered indicators of oxidative stress, may be due to very intense physical exercises, especially with short recovery periods and improper nutrition, should be created (10-13). On the other hand, research has shown that some natural herbal supplements improve health by neutralizing ROS (14). However, the effectiveness of using antioxidant supplements to adapt to exercise in skeletal muscles has been questioned recently (15).
Therefore, the purpose of this article is to investigate the antioxidant adaptation of skeletal muscle tissue caused by aerobic training, the nuclear factor-erythroid 2-related factor 2 (NRF2) signaling pathway, the effect of duration and intensity of aerobic training on oxidative stress, the effect of intermittent and continuous training on oxidative stress in skeletal muscle tissue and the effect of antioxidant supplements and physical exercises on reducing the destructive effects of oxidative stress.
2. Evidence Acquisition
Skeletal muscle is a highly specialized tissue with high flexibility in response to external stimuli such as physical exercise. Repeated muscle contractions during aerobic exercise lead to various physiological adaptations. These responses include the activation of mitochondrial biogenesis, fiber type conversion, and angiogenesis, which increase the muscle's aerobic metabolic capacity and fatigue resistance. In addition to the described effects, aerobic training affects the amount and gene expression of antioxidant enzymes, leading to muscle and cardiovascular system adaptation (15).
Aerobic activity also increases intramuscular triglyceride content, improves insulin sensitivity and muscle glucose absorption, and increases muscle glycogen reserves, reducing the possibility of diabetes and developing muscle function. In this regard, the results of a study showed that aerobic swimming training improves antioxidant defense, which may be related to the higher glycogen content of the skeletal muscles of animals (16).
Recent research shows that ROS has a beneficial role in promoting the physiological adaptation of muscles to aerobic exercise. The physiological production of ROS improves the basic physiological functions at the level of muscle cells, which act as signals for regulation, transmission, proliferation, and transcription. There are many evidences that convince researchers that an optimal or excessive amount of ROS can be the reason for the opposite effects in chronic or acute aerobic activities (3). In this review, 92 articles were studied between 2012 and 2022, of which 54 were used. The search was done in Google Scholar, PubMed, Science Direct, and SID databases. An electronic search of studies was done using ROS, aerobic training, exercise, oxidative stress, antioxidant defense, skeletal muscle, and NRF2. In the next part of this review, we will examine the potential signaling pathway that regulates ROS levels and causes remodeling in skeletal muscle tissue during physical activity.
2.1. Nuclear Factor-Erythroid 2-Related Factor 2 Signaling Pathway
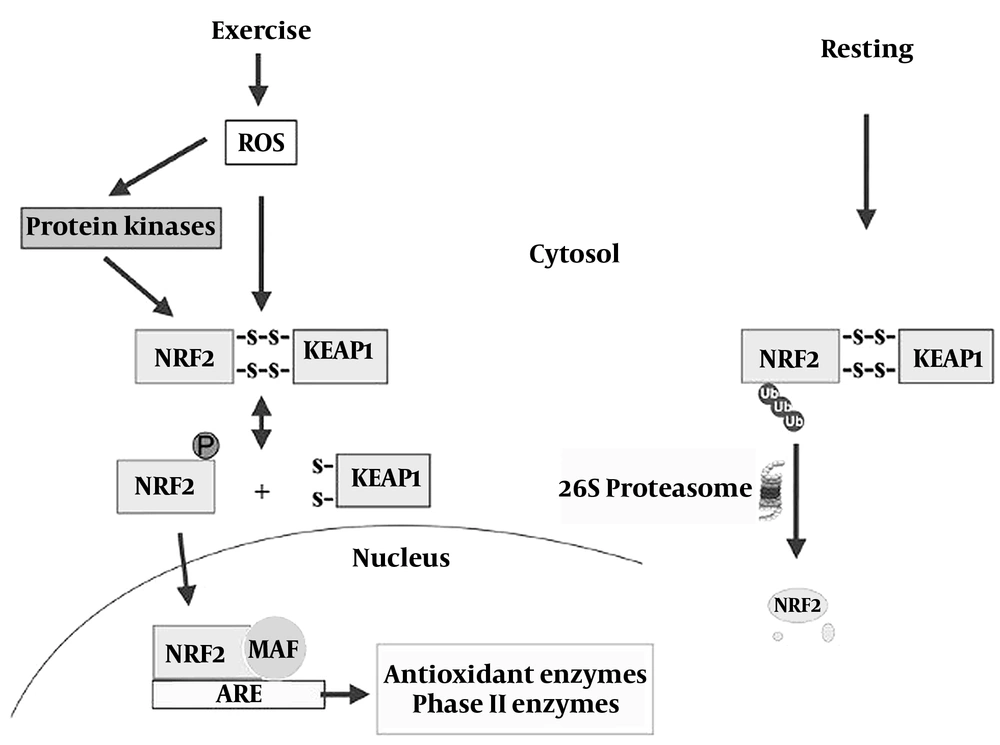
All living organisms have to deal with various stressors during evolution, and only organisms with a functional defense system can survive and evolve. The main pathway of cell protection regulators to endogenous and exogenous stresses caused by ROS compounds is the transcription factor NRF2, which, together with the small proteins of the sarcomere of the aponeurotic muscle fibers (MAF), it binds to the DNA sequence of antioxidant response elements (ARE) of target genes. NRF2 can also bind to inhibitory Kelch-like ECH-associated protein 1 (KEAP1), which can be modified in vitro by various oxidants and electrophiles. The NRF2 system, together with KEAP1, is an anti-stress mechanism that aims to maintain cellular homeostasis (3, 17-19). Thus, under resting conditions, the cellular concentration of NRF2 protein is maintained at very low levels by its inhibitor KEAP1, but under conditions of physical activity and with less availability of free KEAP1, formed NRF2 is accumulated in the nucleus, where it binds to ARE to regulate the transcription of more than 200 genes involved in the antioxidant response, mitochondrial biogenesis, metabolism, detoxification, cell protection, inflammation, autophagy and cell differentiation (Figure 1) (18-20).
However, oxidative stress can activate NRF2 gene expression and transcriptional activity in skeletal muscle cells (in-vitro) or rodent muscle cells (in-vivo). The transcription factor NRF2 is created by reactive oxygen and nitrogen species and is necessary for the adaptive response to physical activity (17, 18).
Research shows that a period of aerobic training significantly increases the expression of the NRF2 gene and decreases the expression of the KEAP1 gene in the muscle tissue of rats. Also, physical exercise, as the main regulator of antioxidant enzymes, activates NRF2 by upregulating the expression of superoxide dismutase (SOD) and decreasing the expression of KEAP1 (21). As a result of the reduction of KEAP1 protein, the ratio of NRF2 to KEAP1 increased significantly, which is related to the significant increase in catalase (CAT) protein (19). Therefore, NRF2 can regulate many antioxidant enzymes, including CAT and SOD (20).
Also, studies have shown that the increase in ROS production caused by periods of acute exercise stimulates the activation of NRF2. For example, an acute exercise session in wild-type mice increases NRF2 gene expression and NRF2 protein abundance in skeletal muscle (22). Of course, regular physical activity leads to the positive regulation of the endogenous antioxidant defense and, in general, a greater capacity to deal with the oxidative damage of biological molecules (17). It has also been found that regardless of the duration and schedule of exercise, regular aerobic physical activity activates NRF2 signaling in skeletal muscle (23) and cardiac muscle (24). Overall, studies show that regular physical activity increases the abundance of NRF2 protein and the number of antioxidant enzymes (17, 18, 20, 21, 23, 24).
3. Results
In general, the production of ROS is a function of the intensity and duration of aerobic exercise because the energy required, the level of oxygen consumption, and the mechanical stress applied to the tissues are different in all types of aerobic exercise (2). Studies have shown that biomarkers of oxidative stress in the blood increase after 60 to 120 minutes of constant-intensity aerobic exercise. In contrast, several studies concluded that regular exercise does not lead to increased oxidative stress in active muscles, which expresses the concept of hormesis caused by aerobic exercise (25, 26). Low levels of ROS production due to aerobic exercise play an important role in skeletal muscle adaptation, and this can be explained by using a hormesis curve, where the optimal level of ROS plays a role in muscle adaptation. At the same time, the amount of ROS higher than the optimal level can lead to various damages to cells and reduce adaptations caused by aerobic training (3, 7, 25-27).
Ryan et al. compared oxidative damage following high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) in the skeletal muscle of rats fed a high-fat diet. Despite large differences in exercise intensity and duration, 12 weeks of HIIT and MICT produced similar acute improvements in insulin sensitivity the day after exercise and similar long-term metabolic adaptations in skeletal muscle in obese adults (28).
In a study, Kawamura et al. also investigated the effect of resistance training with different intensities on the biomarkers of oxidative stress in the plasma and skeletal muscles of rats. The results showed that increasing resistance exercise increases plasma protein carbonyl levels and total antioxidant capacity but does not change skeletal muscle oxidative stress biomarkers in rats (29).
Also, the research investigated the effect of isocaloric aerobic exercise with medium and high intensity on capillary reaction and heart muscle oxidative stress in rats. The results showed that higher exercise intensity caused more improvement in myocardial antioxidant defense, while the increase in capillary reactivity seems to depend more on exercise volume than exercise intensity (30).
Balci and Pepe studied the effect of gender, acute resistance exercise, and regular aerobic exercise on oxidative stress in rats' hearts and skeletal muscles. The results showed that gender is the main factor in changes in the levels of malondialdehyde (MDA) and glutathione (GSH) in the heart and gastrocnemius muscle after endurance exercise or resistance exercise. Also, the response of oxidative stress caused by acute exercise was different in heart muscle tissue and gastrocnemius muscle (31).
On the other hand, the type of activity is an important factor for inducing oxidative damage because the high intensity of cycling reduces oxidative damage by increasing enzymatic antioxidants, but performing high-intensity speed activities increases oxidative damage (32). In this regard, Idrizovic et al. concluded that the optimal intensity of exercise and the type of physical activity might positively affect cardiovascular health indicators in young and healthy women (33). Research results also showed that long-term high-intensity exercise and yoga might be the best choice for reducing oxidative stress in patients with oxidative stress (34).
This shows that the type of exercise and the total volume of exercise play an important role in the oxidative stress caused by physical exercise. It seems that the differences between these studies have been explained by the duration of the training protocol, the type of training, the level of the subjects, and the biomarkers of oxidative damage.
3.1. The Effect of Intermittent and Continuous Exercises on Oxidative Stress in Skeletal Muscles
Studies have found that HIIT and MICT training reduces oxidative stress and inflammatory indicators (35). Thus, MICT can reduce oxidative stress by increasing antioxidant enzymes and reducing ROS production in skeletal muscle, adipose tissue, and vascular tissue (36, 37). Research shows that the activity of antioxidant enzymes CAT and glutathione peroxidase (GPX) in epididymal adipose tissue increased after MICT, while the result of a study showed that HIIT, not continuous training, increases the activity of GPX enzyme in muscle. The underlying mechanism is not fully understood, but HIIT appears to activate redox-sensitive protein signaling pathways through increased ROS production in muscle (35, 38). Ok et al. showed in an obese mouse model that eight weeks of physical activity helps to reduce muscle triglyceride volume by activating lipolysis factors. High-intensity interval training reduces muscle triglycerides more than MICT because greater muscle triglyceride lipolysis results in greater lactate production (39).
In research, Delwing-de Lima et al. evaluated the effect of HIIT and MICT protocols on the changes in oxidative stress parameters caused by a high-fat diet in the blood and liver of rats. Both exercise protocols prevented the increase in carbonyl content and the decrease in CAT. The HIIT protocol increased SOD. Superoxide dismutase did not change in the liver, but protein carbonyl content and CAT increased, and GPX decreased. Moderate-intensity continuous training protocol prevented changes in CAT from a high-fat diet, causing oxidative stress in the blood and liver, and both protocols prevented many changes in oxidative stress parameters (40). Also, the study results showed that both HIIT and MICT protocols decreased blood lipid levels, ROS production, and carbonyl protein content and increased GSH production in the skeletal muscle of mice. Also, both protocols reduced oxidative damage and promoted myokines production response, but specifically, HIIT was more beneficial than MICT in reducing ROS levels in skeletal muscle (41).
Findings support the idea that adaptations to both HIIT and MICT protocols are established in the last exercise session. Also, HIIT and MICT protocols seem to have beneficial but tissue-specific effects on pro/antioxidant status.
Finally, it seems that the adaptations caused by various types of aerobic exercise are limited and can exceed their protective effect and lead to oxidative stress, which the endogenous antioxidant system cannot counter. Whether the use of external (edible) antioxidants in such conditions is useful or not will be investigated further.
3.2. The Effect of Antioxidant Supplements and Exercise on Muscle Adaptations
Today, the reduction of oxidative stress and improving the antioxidant system with antioxidant supplements and exercise training are of great interest (12). Antioxidant supplements neutralize ROS by donating an electron or hydrogen atom and regulate SOD, CAT, and GPX enzymes (13, 42, 43). They also increase the expression of antioxidant enzymes, including those involved in glutathione synthesis, by regulating the NRF2/KEAP1 pathway (14, 44).
In a study, the effect of lemon peel extract and vitamin C supplements on rats that performed exercise was investigated. The results showed that both groups of vitamin C and lemon peel extract significantly increased the levels of CAT and SOD and decreased the levels of MDA (45). Also, a study concluded that just one dose of grape juice two hours before exercise can increase the performance of runners. Grape juice's anti-inflammatory and antioxidant properties can probably lead to better physical activity recovery (46). Ben Dhia et al. also showed in a study that taking melatonin before HIIT, along with modulating oxidative stress and preventing excessive expression of inflammatory mediators, reduces muscle damage in people with obesity (47).
Dutra et al. examined healthy women as subjects to evaluate muscle thickness and function after ten weeks of strength training. They took vitamins E and C during strength training. Peak torque and total work improved during antioxidant supplementation combined with strength training (48). In a study, Taub et al. investigated the relationship between the consumption of antioxidant supplements and increasing the maximum oxygen consumption through the effect on the mitochondria of skeletal muscles and selected healthy and inactive adults for their investigation. They consumed 20 grams of dark chocolate and performed stationary cycling for three months. The results showed that improving mitochondrial function may increase these people's maximum oxygen consumption (VO2max) (49).
However, physical activity and herbal supplements can have synergistic effects. Delfani et al. found in research that aerobic exercise and thistle plant extract can help to improve redox and reduce the oxidative stress of lung tissue (13). However, in a study on old rats, Razavimajd et al. concluded that aerobic physical activity and garlic supplementation separately had a protective effect on the heart tissue of rats, but combined interventions did not have a synergistic effect (50). Kalvandi et al. also showed in research that the performance of elastic resistance exercises in reducing oxidative stress and improving the antioxidant system was more than vitamin D3 supplementation. Vitamin D3 supplementation had no synergistic effect on reducing oxidative stress indices (12). Also, a study investigated the effect of HIIT and orlistat supplementation on adipokines and cytokines. The results showed that although combining HIIT and supplement consumption was significant in many factors, no synergistic effect was observed (51).
Finally, the effect of antioxidant supplementation with exercise training on skeletal muscle adaptation is still unclear. Some background literature suggests that antioxidant supplementation may impair or prevent the signaling of important adaptations such as muscle mitochondrial biogenesis, insulin sensitivity, and hypertrophy. This is consistent with hormesis, where stressors induce ROS, and ROS act as intracellular signaling molecules to promote adaptations that equip the cell to better tolerate subsequent stresses (3, 7, 25-27, 52).
Therefore, it seems that in certain conditions, when athletes are exposed to high oxidative stress or cannot meet antioxidant needs with diet, taking antioxidant supplements may be beneficial (53).
On the other hand, the results of studies showed that antioxidant supplements do not affect exercise adaptation. Trained individuals appear to have a better capacity to buffer oxidants during and after physical activity, and higher levels of ROS input and exercise-induced oxidative stress may be associated with these athletes following redox adaptations to sustained endurance exercise, be useful. Therefore, antioxidant supplements are not recommended for these people (54).
Finally, given that the studies were conducted with recreationally active participants, trained individuals, or even elite athletes, the results may depend on the participant's fitness level, the type of supplement, and the intensity and duration of the exercise.
4. Conclusions
The review of studies conducted in this field showed that aerobic exercise could increase or decrease the gene expression of oxidative damage biomarkers in skeletal muscle, depending on the intensity and duration. Of course, the outcome depends on the specific oxidative damage biomarkers analyzed during the training program.
However, it seems that regular aerobic exercise increases the expression of antioxidants by affecting cellular processes. Therefore, adaptation mechanisms and the reduction of oxidative damage have beneficial effects on skeletal muscle. However, according to the concept of hormesis, the consumption of antioxidant supplements and exercise may disrupt or prevent the signaling of important adaptations such as muscle mitochondrial biogenesis, insulin sensitivity, and hypertrophy. In this way, taking antioxidant supplements before the ROS level reaches the peak of physiological performance can reduce the beneficial effects of physical activity. In contrast, after increasing the ROS level and maximal performance, antioxidant treatment can reduce the destructive effects of oxidative stress in skeletal muscle tissue and improve performance.
Therefore, the increase in oxidative stress caused by exercise training is a function of the amount of ROS production and the capacity of internal antioxidant defense and external (edible) antioxidants, and there is a need for different types of aerobic exercise with different intensities and durations, along with various antioxidant supplements. It should be done for a long time so that better and more reliable approaches can be established for detecting oxidative stress early and preventing the destruction of muscle tissue. Other aspects should also be examined, such as specific oxidative damage biomarkers, reduced stress, and an individual's training status.