1. Background
Cancer is a disease in which cells in the body grow out of control. Cancer is always named for the part of the body where it starts, even if it spreads to other body parts later. When cancer starts in the cervix, it is called cervical cancer (1). Cervical cancer is the fourth most common forms of cancer in women worldwide. The disproportionately high burden of cervical cancer in developing countries and elsewhere in medically underserved populations is largely due to a lack of screening that allows the detection of precancerous and early stage cervical cancer (2). Risk factors for cervical cancer include human papillomavirus (HPV) infection (90% of cases), smoking, a weak immune system, and birth control pills, starting sex at a young age and having many sex partners (3). Several approaches manage the treatment of cervical cancer, including chemotherapy and radiotherapy. However, other strategies with fewer side effects are necessary for treatment and prevention. Relevant approaches particularly food-based entities stay essential in reducing the risk of cancer (4). There are several cell lines for human cervical cancer such as HeLa, C4-1 and SW756. The HeLa cell line is a cervical cancer cell line that is commonly used in experimental research and is aggressive in culture. The HeLa cell line originates from a cervical cancer tumor of a patient named Henrietta Lacks, who died of cancer in 1951 (5). These cells were named “HeLa” after the initial two letters of Henrietta Lacks’ first and last names (6). HeLa cells were the first human cells to be cloned (7). Later research revealed that HeLa cells were robust, immortal and easily propagated over generations in culture (8). HeLa cells grow in vitro so aggressively that they have become problematic and this could be due to its broad adoption—both intentionally and through widespread cross-contamination (9). Herbal plants are bioresources of drugs for traditional systems of medicine, modern medicines, nutraceuticals, food supplements, folk medicines, pharmaceutical intermediates and chemical entities for synthetic drugs (10). Purslane (Portulaca oleracea) is an annual green herb with spinach-like taste. Leaves have an obovate to spatulate form, being 1 - 5 cm long and 0.5 - 2 cm across, while stems are cylindrical, up to 30 cm long and 3 mm in diameter (11). It is a widespread weed, being the eighth most common plants in the world. It can be used as raw salad, as a vegetable dish, or can be cooked or dried to be used with tea or in soup (12). It is reported that the leaves and stems of portulaca oleracea contained ashes, crude protein, lipid and fibers. The stems and leaves have high-energy values about 303.9 Kcal/100g dry weight. Mineral contents are K, Na, Ca, Fe and Zn (13). Purslane has biological properties including antiseptic (14), antispasmodic (15), antibacterial (11), anti-inflammatory (16), antiasthma, and antitussive effects (17). P. oleracea is also used in the treatment of dysentery, skin lesions and insect and snake bite (18). P. oleraceais is listed in the world health organization as one of the most used medicinal plants and it has been given the term “Global Panacea” (19). It is found that the extracts of P. oleracea have inhibitory effects on lipopolysaccharide (LPS) and interferon-γ (IFN- γ) induced NO production (20). Studies show that P. oleracea is a rich source of omega-3 fatty acids, Gallo tannins, kaempferol, quercetin, apigenin and glutathione (21, 22). Nevertheless, its safety and feasibility in clinical trials and the mechanisms by which exerts its antiproliferative property is not clear. Therefore, this study aimed to find the cytotoxicity of ethanolic extract of Purslane on HeLa cell line.
2. Objectives
This study investigates the cytotoxicity and antiproliferative properties of ethanolic extract of Purslane on cervical cancer cell line.
3. Methods
3.1. Preparation of P. oleracea Ethanolic Extract
P. oleracea was purchased from the medical herb garden of Hamadan (Iran) in summer of 2014. Drying at room temperature for two weeks, the plant was powdered. Then the powder of the aerial parts of the plants (stem and leaves) was percolated with 80% ethanol. The volumetric flask was incubated in Ultrasonic bath for two hours. Then, the suspension was filtered through a Bückner funnel for several times. The residues were extracted for 48 and then 72 hours. The filtrations were performed daily until the solution appeared as colorless. The solution was collected in a sterile bottle, then dried in a rotary evaporator at 40°C and stored at -20°C. Concentration of stock was 50 mg/mL, and final work dilutions were made with medium and filtered with 0.22 µm syringe sterile filter.
3.2. Cell Culture
HeLa cell line was obtained from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran).The cells were cultured in RPMI-1640 (Sigma-Aldrich,USA), supplemented with 10% fetal bovine serum (FBS) (Gibco /Invitrogen) and 100 u/ml penicillin - streptomycin (Sigma-Aldrich, USA) in an incubator with 5% CO2 at 37°C with 95% humidity. The culture medium was refreshed every three days. After 80% - 90% confluency, the cells were seeded in 9 six-well plate for MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay. The cells were cultured in six-well plates to evaluate cell survival after treatment with different concentrations of P. oleracea.
3.3. MTT Assay
MTT assay was employed to evaluate the antiproliferative effect of P. oleracea ethanolic extract. Briefly, HeLa cell line was seeded in a flat 9 six-well plate, followed by incubation at 37°C and 5% CO2 in an incubator. After 24 hours, the cells were treated with concentrations of 0 (as control), 300 µg/mL, 500 µg/mL, 700 µg/mL, 1000 µg/mL, 1200 µg/mL and 1500 µg/mL of ethanolic extract of P. oleracea for 24 and 48 hours. Subsequently, 100 μL of the culture medium was removed and 15µL MTT (5 mg/mL) was added to the wells and the plate was incubated for three hours. Then 200 μL of DMSO (dimethyl solfoxide) was added to dissolve the formazan crystals. The absorbance was measured at 570 nm with ELISA reader. Cell proliferation was calculated, using the following formula:
% of alive cells = (OD experimental) / (OD control) × 100
3.4. Cell Viability Assay
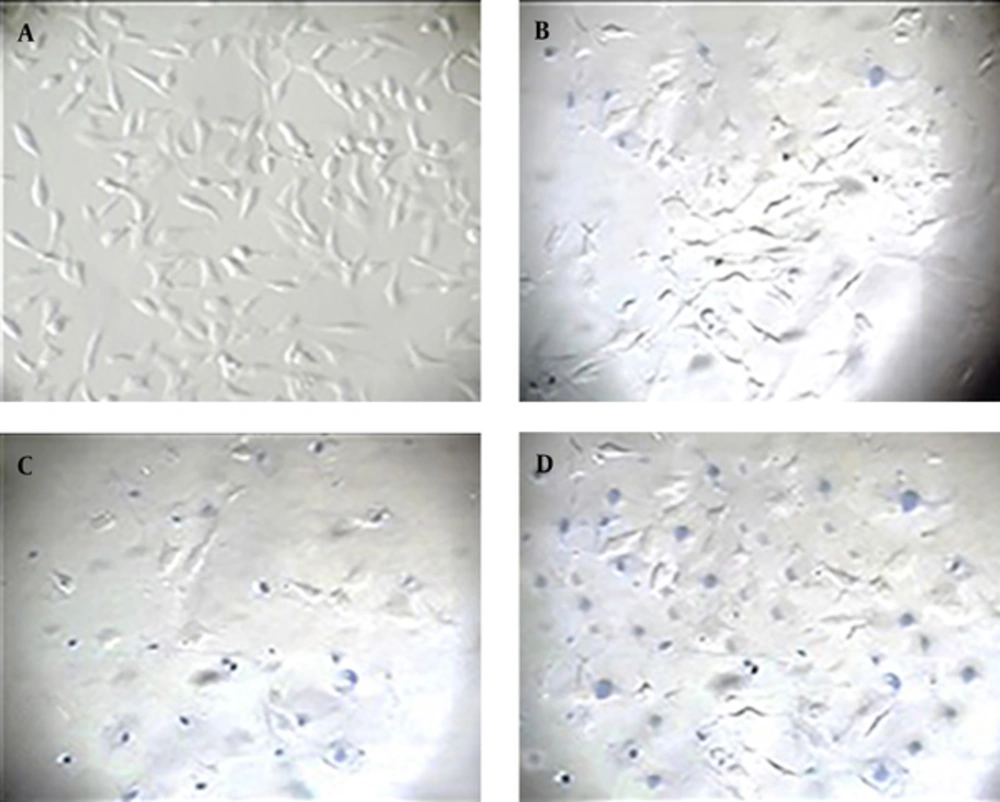
HeLa cell line was seeded in six-well plate and incubated at 37°C in a 5% CO2. The cells were treated with different concentrations of 700, 1000, 1200 and 1500 µg/mL of P. oleracea ethanolic extract for 24 and 48 hours. The doses induced 50% or more cell death with MTT assay and were selected for trypan blue staining. The culture medium was removed and replaced with trypan blue (0.4%), and kept for 3-5 minutes. The cells were washed three times with PBS and observed under a microscope. Blue color cells represent as dead cells. Viability rate was measured according to the below formula:
Cell viability (%) = (Live cells) / (Total number of cells) ×100
3.5. Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical comparisons were analyzed by one-way analysis of variance, using Tukey test for multiple comparisons (SPSS software Version 22). Differences were considered significant at P < 0.05.
4. Results
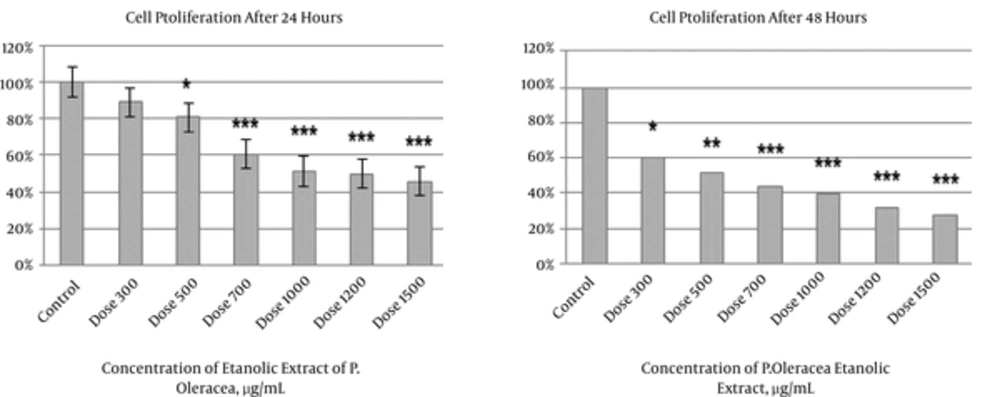
4.1. P. oleracea Extract Effectively Decreased Cell Proliferation
The results of MTT assay indicated that the ethanolic extract of P. oleracea showed an inhibitory effect on proliferation in all doses on HeLa cell line compared to the controls after 24 and 48 hours. It was found that with an increase in the dose, a higher decline occurred in proliferation. In addition, it seems that P. oleracea acts in a time dependent manner so that less proliferation was detected after 48 hours. 1500 μg/mL P. oleracea induced the most cytotoxicity on HeLa cells after 48 hours (P < 0.001). In addition, the same result was obtained with 1500 μg/mL after 24 hours, showing the most cytotoxic effects on the cells (P < 0.001). With 700, 1000, and 1200 μg/mL of P. oleracea, 50% or more cells died (Figure 1).
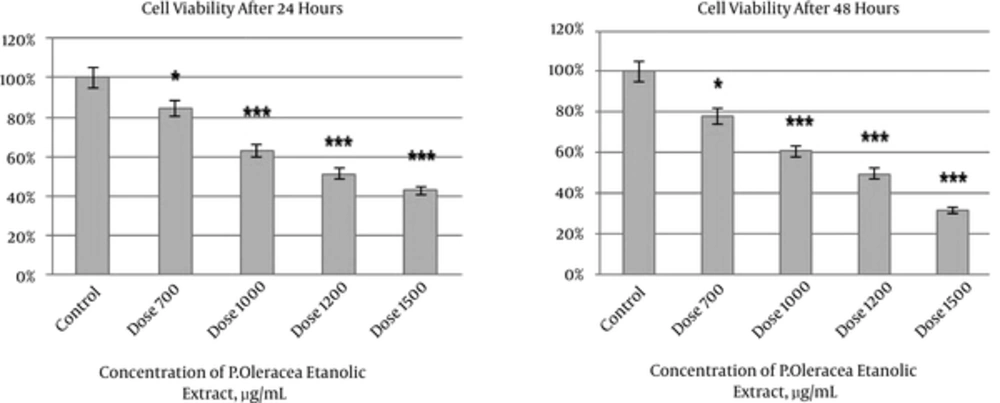
4.2. Effects of P. oleracea Extract on Cell Viability
Our results revealed that 700, 1000, 1200, and 1500 µg/mL of ethanolic extract P. oleracea significantly reduced the survival rate of HeLa cells after 24 and 48 hours (P < 0.001). A relationship was detected between the reduction of cell viability and increasing concentrations of ethanolic extract. The lowest cell survival rate occurred after administration of 1500 µg/mL (P < 0.001). The more cell death was observed after 48 hours compared to 24 hours. The findings obtained with trypan blue confirmed our previous MTT results. P. oleracea exerts its effects in a dose and time- dependent manner (Figures 2 and 3).
5. Discussion
Application of supplementary agents in cancer treatment is encouraging and developing increasingly. This study confirmed that P. oleracea ethanolic extract efficiently present its antitumor effects through inhibition of growth and survival of HeLa cells. Interestingly, it acts in a time and dose dependent manner. It has been shown that P. oleracea aqueous extract exhibits antiproliferative and apoptotic effects against HeLa cell line in a dose and time dependent manner (23). In a study conducted by Payudara et al. methanolic extract of P. oleracea had growth inhibition on human breast MCF-7, cervical HeLa, colon HT-29 and nasopharyngeal CNE-1 cancer cell lines, compared with normal liver cells. They showed that the plant extract is non-toxic for normal cells and is safe for daily consumption (24). Some studies have shown that active constituents of P. oleracea ethanol extract have the cytotoxic, apoptotic, antiproliferative and cell cycle arrest properties against two AMN3 (Murine mammary adenocarcinoma) and RD (Rabdomyosarcoma) cancer cell lines after 24, 48 and more efficiently 72 hours (25). It has been confirmed that after increasing the concentration of aqueous extract of P. oleracea to 100 μg /ml, more HEPG2 (liver hepatocellular carcinoma) cells faced death, and these results showed the cytotoxic effect of P. oleracea on this cell line (26). The antiproliferative and antiapoptotic properties of the extract might explain its bioactive components, including flavonoids, alkaloids and anthraquinones. Several flavonoids regulate the genes, which are critical for the control of proliferation, cell cycle and apoptosis pathway in cancer cells (27). It has been found that Portulaca oleracea seed oil can decrease the cell viability of both HepG2 (human liver cancer) and A-549 (human lung cancer) cell lines in a concentration-dependent manner (28). Some flavonoids, including apigenin based, inhibit the formation of cyclooxygenase-2 (COX-2), and thus playing an important role in preventing cancer and inflammation in part through inhibiting COX-2 enzymes (29). COX-2, as an inducible enzyme, is associated with inflammation (30). Luteolin as a flavonoid in P. oleracea is able to induce cell cycle arrest and apoptosis of colon cancer HT-29 cell through a decrease of IGF-II production and down regulation of insulin-like growth factor-I receptor signaling (12, 31). Fatty acid may have an important role in the protection against cardiovascular disease and cancers (12). P. oleracea contains large amount of dopamine and acts as an antitumor agent. Dopamine may inhibit the production or release of endogenous factors required for cell viability and proliferation (32) and may inhibit the vascular endothelial growth factor/vascular permeability factor (VPF/VEGF) induced angiogenesis by acting on D2 dopamine receptors present on endothelial cells (33). It has been reported that water-soluble polysaccharides isolated from P. oleracea have mild cytotoxic activity against cervical cancer HeLa cell line. In addition, sulphated form of these polysaccharides improves its antitumor effect (34). A significant increase of HeLa cells arrested at S phase and undergoes apoptotic phenomenon was detected when cells were treated with P. oleracea sulphated-polysaccharides (35). It has been indicated that POL-P3b possesses the activity of inhibiting cervical cancer cell growth in vitro and in vivo at a concentration and time-dependent manner (36). POL-P3b (water-soluble polysaccharide) is a polysaccharide fraction purified from Portulaca oleracea L. and it is able to enhance immunity and inhibit tumor formation (37). Moreover, it was found that P. oleracea methanol extract was able to reduce DNA synthesis that was detected in BrdU (5-Bromo-2’-deoxyuridin) assay and arrest both CNE-1 and HeLa cell under S phase of the cell cycle (24, 38). However, the mechanism of this bioactive molecule is still unclear, and some issues about its optimal concentration and exposure duration, its efficiency compared to routine cancer therapy medicines and its behavior against normal cells, must be studied to understand the potential of P. oleracea as a chemopreventive food.
5.1. Conclusion
In conclusion, our finding indicated that P. oleracea ethanolic extract has antitumor activity against HeLa cell line in a dose and time dependent manner after 24 and 48 hours. We suggested that this extract might be considered as a therapeutic candidate in cancer therapy. Nevertheless, more examination should be done in vitro and preclinical settings before conducting clinical trials; and its efficiency on normal cells should be clarified in future studies; and this will be done by our research team in the near future.