1. Background
Diabetes mellitus is a metabolic disorder characterized by various complications, such as increases in blood glucose and disturbances in the metabolism of carbohydrates, fat, and protein (1). According to the predictions of the world health organization (WHO), diabetes mellitus will affect 366 million adults globally by 2030 (2, 3). This disease is classified into two major types: type 1 diabetes, which is caused by a loss of pancreatic ability to secrete insulin, and type 2 diabetes, which is caused by several factors including resistance to insulin performance and inappropriate response of the insulin secretary (4, 5). Patients with T2D who adopt a lax attitude toward their disease may suffer in future from several complications of T2D, such as disturbances of the eyes, kidneys, and nervous system in the form of retinopathy, nephropathy, and neuropathy, respectively (6, 7). Several studies have determined the risk factors for the development of T2D, which include obesity (3, 8), depression (9), sleeplessness (10), high serum uric acid (11), smoking (12), and alcohol (13).
Various reports have indicated some gene polymorphisms, such as those associated with the ATP-binding cassette transporter A1 (ABCA1), as risk factors for T2D. ABCA1 is a member of a large family, called the ABC family, which includes 49 mammalian trans-membrane transporters (14). ABCA1 has 2261 amino acids that occur between the cell membrane as an integral protein and the human ABCA1 gene on chromosome 9q31 (15). This transporter suppresses the inflammation response of macrophages and exports cholesterol and phospholipids from cells. However, this transporter also exports other substances, including α-tocopherol, interleukin-1β, and apolipoprotein E (ApoE) (14). ATP serves as a source of energy for these processes (16). ABCA1exports cholesterol by a multistep pathway that include the formation of a cell-surface lipid domain, binding of apolipoproteins to this transporter, and activation of signaling processes after lipid solubilization by the apolipoproteins (17). ABCA1, like other transporters that utilize ATP, has Walker A and Walker B peptide motifs, and a unique amino acid signature exists between these Walkers (16). The latest studies have shown that ABCA1 is associated with different diseases, such as high-density lipoprotein deficiency, familial HDL deficiency, Tangier disease (16), cardiovascular diseases, and T2D (14).
People with T2D also have metabolic issues that include problems with lipid metabolism. ABCA1 acts as a transporter in the transition of lipids, especially cholesterol, and it is further associated with the abnormalities of serum lipid levels of high-density lipoprotein (HDL) observed in T2D cases. This condition is thought to be involved in the mechanism of insulin resistance as a cause of T2D (14).
2. Objectives
The aim of the present study was to investigate, for the first time, the association between ABCA1 polymorphisms and the development of T2D in an Iranian population (Southeastern Iran, Zahedan).
3. Methods
The present investigation was conducted at the Ali-Asghar hospital in Southeastern Iran. The participants were 250 T2D patients and 250 healthy individuals. Demographic data, including age, gender, and body mass index (BMI), were collected through interviews. T2D was diagnosed when patients had fasting blood sugar (FBS) levels greater than 126 mg/dL, HbA1c ≥ 6.5%, confirmed by at least two endocrinologists. Individuals selected for the control group had to have no specific systemic diseases and no family relationship with the T2D group members. The study was supported financially by Zahedan University of Medical Science, and the Ethics committee of Zahedan University of Medical Sciences approved the protocol of this study. Participants consented to take part in this study.
3.1. DNA and Clinical Analysis
Two milliliters of peripheral blood (venous vessel) in tubes containing anticoagulant ethylenediaminetetraacetic acid (EDTA) were taken from each member of both groups; these tubes were then used for extraction of DNA by a salting out method (18, 19). Clinical data consisted of fasting blood sugar (FBS), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) (in serum from both groups)
3.2. A/G Polymorphism of the ABCA1 Gene (rs4149313)
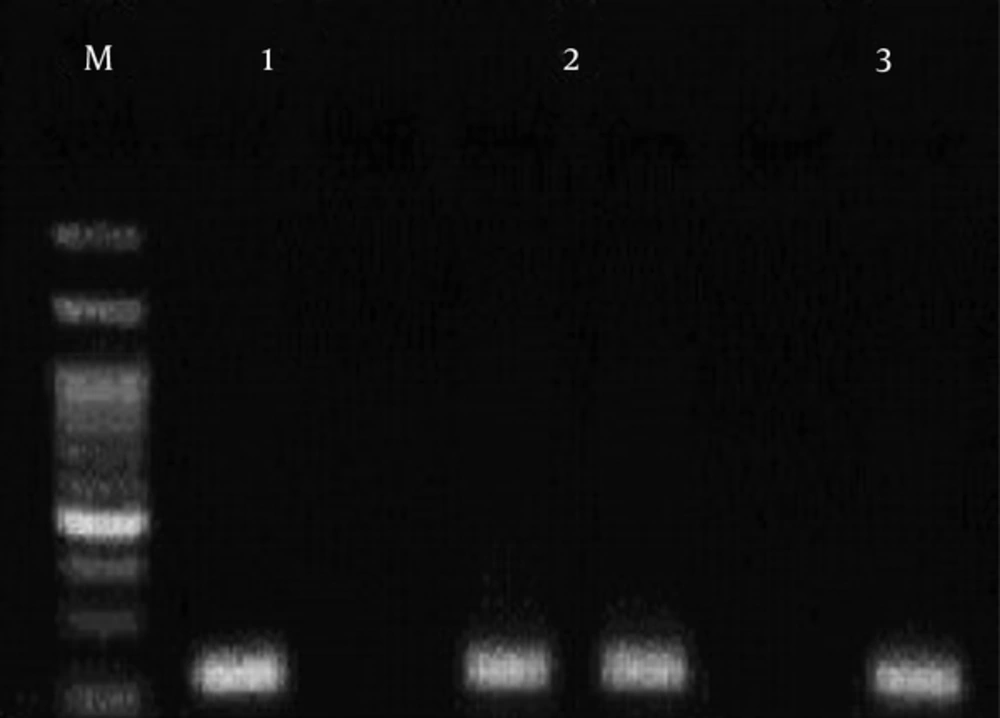
This region of ABCA1 gene was amplified using three oligonucleotide primers (Table 1). Primers were designed for amplification-refractory mutation system (ARMS)-PCR with the following conditions: an initial denaturation at 95°C for 5 minutes, followed by 30 cycles with these conditions: denaturation at 95°C for 30 seconds, annealing at 49°C for 30 seconds, extension at 72°C for 29 seconds, and final extension at 72°C for 5 minutes. The sizes of the PCR products were 223 base pairs (bp) for rs4149313 (Figure 1).
| Primer 5’ - 3’ | Product | Method |
|---|---|---|
| rs4149313 | 223 bp | ARMS |
| Fw: TGGTTCCAACCAGAAGAGAAGA | ||
| FM: TGGTTCCAACCAGAAGAGAAGG | ||
| R: GAACTCCCAATAGGTCAACA | ||
| rs2230806 | 319 bp | ARMS |
| Rw: CTGCTGCAGCCAGTATCTCAT | ||
| RM: CTGCTGCAGCCAGTTTCTCAC | ||
| F: GCCTTGTGCTATGATGCATT |
3.3. A/G Polymorphism of the ABA1 Gene (rs2230806)
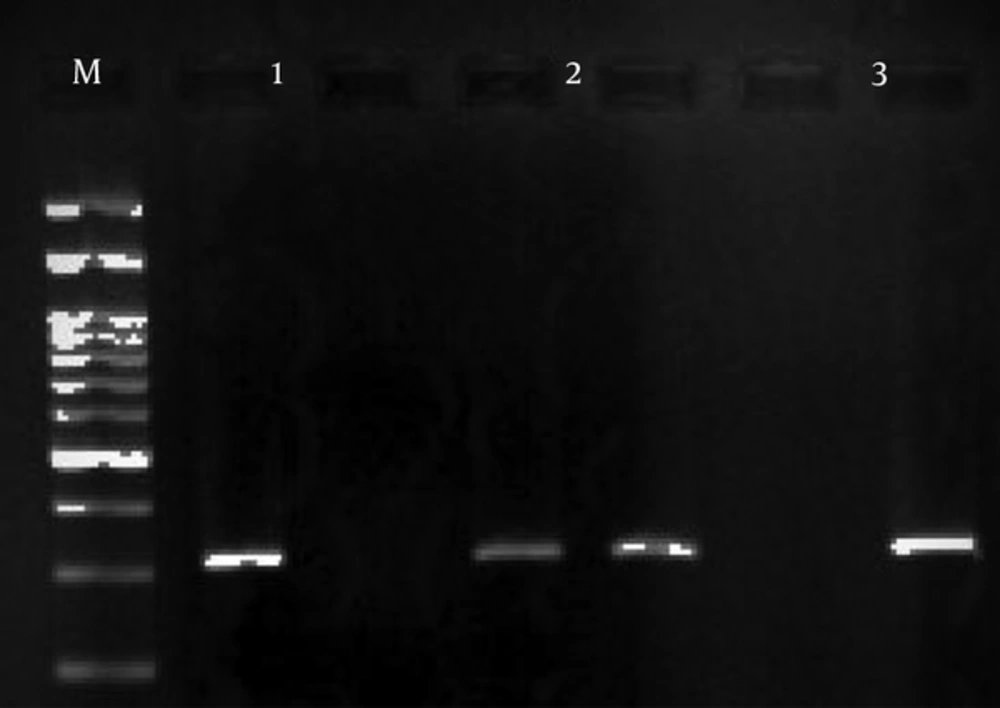
Three primers were designed to amplify this region of the ABCA1 gene (Table 1). The ARMS-PCR method was used to compare the polymorphism between the T2D and control groups. The PCR conditions started with an initial denaturation at 95°C for 5 minutes, and then 30 cycles of PCR with the following conditions in every cycle: denaturation at 95°C for 30 seconds, annealing at 48°C for 30 seconds, and extension at 72°C for 29 seconds. The final step was extension at 72°C for 5 minutes. The PCR product size was 319bp (Figure 2).
The PCR products were detected following electrophoresis on a 1.5% Agarose gel containing ethidium bromide and viewing under UV light (Figures 1 and 2).
3.4. Statistical Analysis
SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis of data. The Student’s t-test was used for comparing quantitative variants of both groups (T2D patients and controls). The Fisher’s exact test or χ2-test was used for analysis of genotypes and frequencies of the data. The calculation frequency of alleles was done by the gene counting method. The 95% confidence intervals (95% CI) and odds ratio were estimated via logistic regression. PHASE software version-2.1 was used to calculate the frequency of haplotypes. The Hardy–Weinberg equilibrium (HWE) was examined using χ2 for each single nucleotide polymorphism (SNP). Multivariate logistic regression models were used to evaluate the associations between T2D and ABCA1 genotypes. Statistical significance for all data was considered at P < 0.05.
4. Results
This study consisted of 250 patients with T2D with mean age of 54.87 ± 10.13 years and 250 health people with mean age of 48.86 ± 10.01 years. The clinical and demographic characteristics of T2D patients and controls are shown in Table 2. There was not any significant difference in age and gender between the T2D patients and the control group (P > 0.05). There was a significant difference between the LDL of the T2D patients and controls (P = 0.023), also there was as a significant difference in BMI and FBS (P < 0.0001) between these groups. Also, the result of multivariate logistic regression was used to adjust for potential confounding factors including BMI, gender, age, FBS, TC, TG, HDL, and LDL for both SNPs (Table 2).
| T2D (n ± SD) | Controls (n ± SD) | P Value | |
|---|---|---|---|
| Age (years) | 54.87 ± 10.13 | 48.86 ± 10.01 | 0.593 |
| Sex(Female/male) | 183/67 | 174/76 | 0.429 |
| FBS (mg/dL) | 188.40 ± 86.45 | 97.70 ± 19.21 | < 0.0001 |
| TC (mg/dL) | 183 ± 44.31 | 181.84 ± 36.1 | 0.752 |
| TG (mg/dL) | 161.40 ± 83.24 | 148.1 ± 94.59 | 0.100 |
| HDL (mg/dL) | 55.4 ± 20.17 | 54.01 ± 14.68 | 0.427 |
| LDL (mg/dL) | 97.21 ± 34.33 | 104.49 ± 29.06 | 0.023 |
| BMI (kg/m2) | 27.63 ± 5.49 | 21.48 ± 2.43 | < 0.0001 |
| Genotypesars4149313 | P Value (Odds Ratio (95% CI)a) | Genotypesa rs2230806 | P Value (Odds Ratio (95% CI)a) |
| AA | Ref | AA | Ref |
| AG | 0.760 (0.848 (0.295 - 2.441)) | AG | 0.696 (0.509 (0.017 - 15.024)) |
| GG | 0.957 (0.963 (0.238 - 3.896)) | GG | 0.853 (0.809 (0.086 - 7.574)) |
| BMI | < 0.0001 (0.506 (0.412 - 0.621)) | BMI | < 0.0001 (0.505 (0.412 - 0.619)) |
| Sex | 0.690 (0.830 (0.331 - 2.079)) | Sex | 0.721 (0.843 (0.331 - 2.147)) |
| Age | 0.001 (0.923 (0.880 - 0.968)) | Age | 0.001 (0.924(0.881- 0.969)) |
| FBS | < 0.0001 (0.960 (0.946 - 0.973)) | FBS | < 0.0001 (0.960 (0.946 - 0.973)) |
| TC | 0.538 (0.992 (0.967 - 1.018)) | TC | 0.521 (0.992 (0.967 - 1.017)) |
| TG | 0.911 (1.000 (0.993 - 1.006)) | TG | 0.913 (1.000 (0.993 - 1.006)) |
| HDL | 0.540 (1.011 (0.977 - 1.046)) | HDL | 0.553 (1.010 (0.976 - 1.046)) |
| LDL | 0.936 (1.001 (0.974 - 1.030)) | LDL | 0.915 (1.002 (0.974 - 1.030)) |
Abbreviations: BMI, Body Mass Index; FBS, Fast blood sugar; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; TC, Total Cholesterol; TG, Triglyceride.
aMultivariate logistic regression was used to adjust for potential confounding factors including BMI, sex, age.
Genotype and allele frequencies of ABCA1 (rs4149313 and rs2230806) polymorphisms in T2D patients and controls are shown in Table 3. The frequencies of genotypes and alleles in rs4149313 polymorphism of ABCA1 gene showed significant differences between genotype GG (OR = 2.41, %95 CI=1.41 - 4.11, P < 0.001) and allele G (OR = 1.67, %95 CI = 1.25 - 2.23, P < 0.0001) of the T2D patients and controls. Frequencies of rs2230806 polymorphism showed no significant difference of allele G and genotype GG between the T2D patients and the controls. Statistical analysis of the clinical and demographic data in dominant (AG + GG vs. AA in the rs4149313 polymorphism), showed that age in T2D patients group and BMI in the controls were significantly different. In evaluating rs2230806 polymorphism (AG + GG vs. AA), in the T2D patients group, gender and BMI were significantly different, and in the controls group, age and TG were significantly different (Tables 4 and 5).
| Genotype | T2D (n = 250) | Controls (n = 250) | P Value | OR (95% CI) |
|---|---|---|---|---|
| ABCA1 (rs4149313) | ||||
| AA, n (%) | 150 (60) | 172 (68.8) | Ref = 1.00 | |
| AG, n (%) | 50 (20) | 54 (21.6) | 0.808 | 0.947 (0.608 - 1.475) |
| GG, n (%) | 50 (20) | 24 (9.6) | < 0.001 | 2.41 (1.41 - 4.11) |
| Allele | ||||
| A, n (%) | 350 (70) | 398 (79.6) | 1.00 | |
| G, n (%) | 150 (30) | 102 (20.4) | < 0.0001 | 1.67 (1.25 - 2.23) |
| Dominant | ||||
| AA | 150 (60) | 172 (68.8) | 1.00 | |
| AG + GG | 100 (40) | 78 (31.2) | 0.04 | 1.47 (1.02 - 2.13) |
| Recessive | ||||
| AA + AG | 200 (80) | 226 (90.4) | 1.00 | |
| GG | 50 (20) | 24 (9.6) | 0.001 | 2.38 (1.39 - 4.00) |
| Overdominant | ||||
| AA + GG | 200 (80) | 196 (78.4) | ||
| AG | 50 (20) | 54 (21.6) | 0.66 | 0.91 (0.59 - 1.39) |
| ABCA1 (rs2230806) | ||||
| AA, n (%) | 10 (4) | 10 (4) | 1.00 | |
| AG, n (%) | 9 (3.6) | 10 (4) | 0.872 | 0.765 (0.245 - 2.70) |
| GG, n (%) | 231 (92.4) | 230 (92) | 0.555 | 1.077 (0.437 - 2.651) |
| Allele | ||||
| A, n (%) | 29 (5.8) | 30 (6) | 1.00 | |
| G, n (%) | 471 (94.2) | 470 (94) | 1.00 | 1.037 (0.61 - 1.75) |
| Dominant | ||||
| GG | 231 (92.4) | 230 (92) | 1.00 | |
| AG + AA | 19 (7.6) | 20 (8) | 0.87 | 0.94 (0.49 - 1.81) |
| Recessive | ||||
| GG + AG | 240 (96) | 240 (96) | 1.00 | |
| AA | 10 (4) | 10 (4) | 1.00 | 1.00 (0.41 - 2.45) |
| Overdominant | ||||
| GG + AA | 241 (96.4) | 240 (96) | ||
| AG | 9 (3.6) | 10 (4) | 0.82 | 0.89 (0.36 - 2.22) |
Abbreviations: CI, confidence interval; OR, odds ratio.
| Genotype | Age (Year) | Sex (Female/Male) | BMI (kg/m2) | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|---|---|---|
| rs4149313 | |||||||
| AA | 54.47 ± 10.98 | 106(F)/44(M) | 27.53 ± 4.57 | 185.13 ± 48.10 | 166.95 ± 86.53 | 55.89 ± 20.90 | 99.90 ± 36.76 |
| AG+GG | 55.48 ± 8.72 | 77(F)/23(M) | 27.78 ± 6.61 | 179.73 ± 37.77 | 152.87 ± 77.58 | 54.64 ± 19.08 | 93.07 ± 29.96 |
| P-value | 0.035 | 0.268 | 0.296 | 0.209 | 0.238 | 0.282 | 0.399 |
| rs2230806 | |||||||
| AA | 58.9 ± 10.32 | 4(F)/6(M) | 30.83 ± 15.05 | 178.1 ± 24.31 | 145.20 ± 73.31 | 55.72 ± 15.30 | 98.16 ± 21.71 |
| AG+GG | 54.70 ± 10.11 | 179(F)/61(M) | 27.47 ± 4.60 | 183.21 ± 44.97 | 162.07 ± 83.70 | 55.38 ± 20.40 | 97.16 ± 34.84 |
| P-value | 0.799 | 0.016 | 0.001 > | 0.157 | 0.79 | 0.655 | 0.408 |
Abbreviations: BMI: Body Mass Index, FBS: Fast blood sugar, TC: Total Cholesterol, TG: Triglyceride, HDL; High-density lipoprotein, LDL: Low-density lipoprotein
| Genotype | Age (Year) | Sex (Female/Male) | BMI (kg/m2) | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) |
|---|---|---|---|---|---|---|---|
| rs4149313 | |||||||
| AA | 48.36 ± 9.81 | 121 (F)/51 (M) | 21.53 ± 2.33 | 181.48 ± 36.64 | 146.29 ± 95.57 | 53.12 ± 14.91 | 103.20 ± 29.54 |
| AG+GG | 49.93 ± 10.43 | 53 (F)/25 (M) | 21.4 ± 2.67 | 182.60 ± 35.18 | 151.68 ± 93.08 | 56.00 ± 14.07 | 107.27 ± 28.07 |
| P-value | 0.782 | 0.702 | 0.034 | 0.514 | 0.607 | 0.265 | 0.775 |
| rs2230806 | |||||||
| AA | 49.10 ± 19.01 | 7 (F)/3 (M) | 22.20 ± 3.08 | 184.11 ± 24.59 | 146.4 ± 178.30 | 56.29 ± 13.67 | 100 ± 27.21 |
| AG+GG | 48.85 ± 9.53 | 167 (F)/73 (M) | 21.46 ± 2.41 | 181.75 ± 36.51 | 148.15 ± 89.85 | 53.92 ± 14.75 | 104.68 ± 29.20 |
| P-value | 0.015 | 0.978 | 0.082 | 0.440 | 0.048 | 0.555 | 0.884 |
Abbreviations: BMI, body mass index; FBS, fast blood sugar; HDL, high-density lipoprotein; LDL, low- density lipoprotein; TC, Total Cholesterol; TG, Triglyceride.
The frequency rates of four common haplotypes of two SNPs in ABCA1 gene (rs4149313 and rs2230806) are shown in Table 6. Although there was no significant difference between AA and GA haplotypes and T2D (P = 0.37 and P = 0.4, respectively), GG haplotype was significantly different between T2D patients and controls (P = 0.0014, OR = 1.52, 95%CI = 1.18 - 1.96) as risk factors for T2D. Finally, All the loci were conducted to the Hardy-Weinberg equilibrium (HWE) in both T2D patients and controls. In the T2D patients or controls, the genotype distribution of both SNPs rs4149313 (controls; P < 0.001, χ2 = 28.04, Case; P < 0.001, χ2 = 68.59) and rs2230806 (controls; P < 0.001, χ2 = 104.13, Case; P < 0.001. χ2 = 112.41) was not in HWE.
| Rs4149313 | Rs2230806 | T2D (250) | Controls (250) | P Value | OR (95% CI) |
|---|---|---|---|---|---|
| A | G | 0.6527 | 0.7532 | 1.00 | |
| G | G | 0.2893 | 0.1868 | 0.0014 | 1.52 (1.18 - 1.96) |
| A | A | 0.0473 | 0.0428 | 0.37 | 1.25 (0.76 - 2.08) |
| G | A | 0.0107 | 0.0172 | 0.4 | 0.63 (0.21 - 1.85) |
Abbreviations: CI, confidence interval; OR, odds ratio.
5. Discussion
In this study, two single nucleotide polymorphisms (SNP) were investigated in genes related to cholesterol efflux from cells (ABCA1) in T2D. The G allele of rs4149313 of ABCA1 gene was indicated as a risk factor for T2D, while the G allele of rs2230806 of this gene did not differ significantly between the patients with T2D and the controls. T2D an inflammatory and chronic disease (20) that involves different organs, but especially the nervous system, kidneys, and eyes (6). This disease leads to metabolic problems in the metabolism of lipids, and an association exists between ABCA1 and an enhanced risk of T2D (14). Fan et al., in their meta-analysis, showed that the rs4149313 polymorphism of the ABCA1 gene is probably associated with coronary heart disease and is a risk factor in Asian populations. Coronary heart disease is one of the complications of uncontrolled diabetes and is another inflammatory disease, like diabetes (21). Yamaguchi et al. investigated the rs4149313 polymorphism of the ABCA1 gene in a Japanese population and found a significant association with T2D (22). By contrast, Wang et al. studied the rs2230806 polymorphism and found no significant association between this SNP of the ABCA1 gene and the risk of T2D (23).
ABCA1 plays an important role in lipid flow, and particularly in cholesterol efflux from cells, due to its role in the regulation of high-density lipoprotein (HDL) levels (24). HDL is one lipid profile usually screened; others include total cholesterol, very low-density lipoprotein, low-density lipoprotein, and triglycerides (25). Several SNPs of the ABCA1 gene have apparent associations with alterations of the lipid profile (26).
In the present study, there was no statistically significant difference between rs2230806 genotypes of the controls and TGs (P = 0.048). This result agreed with the findings of Kolovou et al. on young Greek nurses, in which blood lipid levels were not associated with the rs4149313 or rs2230806 polymorphisms of the ABCA1 gene (27). Marraki et al. also observed no statistical association between either SNP and the lipid profile in a Greek population (28).
The association between ABCA1 polymorphism and body mass index in T2D patients was also assessed. BMI is a value derived from the weight and height of an individual. The normal range is between 18.5 - 25 (29). A BMI difference was observed between the patient and control groups (P < 0.0001), also the BMI was statistical difference between the genotypes of the two SNPs. This parameter showed a significant difference in the rs2230806 polymorphism between the AA genotype and AG + GG genotypes in the T2D group (P < 0.0001). The BMI in the controls also differed between genotype AA and genotype AG + GG for either rs4149313 or rs2230806 (P = 0.034 and P = 0.082, respectively). Haghvirdizadeh investigated the ABCA1 rs2230806 polymorphism in a Malaysian population and found that this SNP differed significantly between patients with T2D and the controls in terms of BMI (30). In another study, Flores-Dorantes showed a significant difference between the R230C gene variant of ABCA1and BMI (31). Lu et al., who examined a Han Chinese population in Taiwan, found that the BMI of persons with T2D and the rs2230806 polymorphism differed significantly from those who had different genotypes (32).
The explanation for the observed deviation from HWE is not clear in the present population; it may have arisen for several reasons, such as small sample size, migration, or the consanguineous marriages common in this part of the country (Southeastern Iran, Zahedan).
In conclusion, the G allele of rs4149313 of the ABCA1 gene was associated with T2D as a risk factor in the study population from Southeastern Iran. This association may be due to the role of ABCA1 in exporting lipids from cells and its suppressant role in the inflammation response associated with T2D, as in other inflammatory diseases, and to the alteration of lipid metabolism. Further investigations with larger samples are required to confirm this association.