1. Context
According to the U.S. Department of Health & Human Services, nearly 107,000 of U.S. people needed a transplant to survive in 2020, while only ∼ 35% of them received it (1).
Application of autologus intestinal segments is a method commonly used in the clinics for bladder reconstruction. Ileal or bladder tissue or nephrectomy have been also utilized for the ureter replacement. These surgical techniques are associated with deleterious effects such as bladder stone formation, urinary incontinence, mucus formation, stenosis, and infection (2, 3).
Therefore, developing a new technique to reconstruct the urinary tract takes on an added importance in modern urology. Several studies have been conducted to develop tissue engineering methods for replacing urinary tract tissues. Progressions in tissue engineering can solve the complications of conventional therapeutic methods and restore normal functions of tissues (4). The basis of this method is in vitro seeding of stem cells on a biodegradable scaffold in order to attain urinary tract regeneration. Then this constructed graft is transplanted to the defect site of the body. This study aimed to investigate cell sources, different scaffolds, and protocols used in urinary tract tissue engineering.
2. Cell Sources
It is known that cell-free scaffolds can induce fibrosis post-operatively (5, 6). On the other hand, according to the research, using cells can prevent scar formation and enhance tissue regeneration (7, 8). Tissue specific autologous cells were obtained from an individual, cultured and expanded in vitro, seeded on scaffold, and implanted into a defect site for tissue regeneration.
2.1. Autologous Urothelial Cells
These cells are commonly isolated from a bladder biopsy. However, this procedure may require surgery and cause trauma to the bladder. Therefore, some researchers have used urine and bladder washes to obtain urothelial cells (UCs). These cells have potential to regenerate urinary tract wall. The characteristics of isolated cells from urine are similar to those of normal bladder cells (9).
2.2. Autologous Epidermal Cells
These cells can be readily isolated from foreskin. They were successfully seeded on acellular collagen matrix and transplanted to the urethral defect in rabbits. Results showed normal urethral diameter without any strictures. Also, transitional cell layer was seen in histological sections (10).
2.3. Autologous Epithelial Cells
As buccal keratinocytes and lingual keratinocytes are great sources for urinary tract regeneration due to their structural similarity to urothelium. However, a biopsy from buccal mucosa can result in donor morbidity (11).
2.4. Stem Cells
These cells are undifferentiated and self-renewing cells that can be used to regenerate a wide range of urinary tract clinical problems (12). The application of adult stem cells, compared to embryonic stem cells (ESCs), is preferred for therapy due to the absence of ethical issues and lower risk of tumor formation after transplantation (2). Grafts, composed of stem cell seeded biomaterials, could result in urinary tract reconstruction more than decellularized extracellular matrices in porcine and canine bladder models (13).
Adult stem cells (SC) can be extracted from the specific organs such as adipose tissue, bone marrow, skin, and blood (14). Mesenchymal stem cells (MSCs) are most often used for experimental cell-based therapy in urinary tissue engineering. It enjoys good protocols for MSC extraction, has high proliferation potential, and has no tumor formation and MSC capability to differentiate various tissues (2).
Bone marrow mesenchymal stem cells (BMSCs) have the potential to differentiate both smooth muscle cells (SMCs) and UCs of the urinary tract wall. However, their application is limited due to the low content of MSC in BM, the invasive extraction method, and long-term in vitro propagation. It seems that adipose-derived mesenchymal stem cells (ADSCs) are better choices because they do not have the above limitation (15). Human ADSCs were differentiated into SMCs by incubation in smooth muscle inductive media and seeded on synthetic urinary bladder composite. Then cell seeded scaffolds were implanted in nude rats after partial cystectomy. These grafts maintained the bladder capacity and compliance (16).
Human umbilical cord-derived MSCs (hUMSCs) are extracted from the cord blood. Human umbilical cord-derived MSCs could probably be a privileged source for urinary tract repair, due to their multiple differentiation ability and less immunogenicity compared to other sources of adult stem cells (17, 18).
Urine-derived SC (USCs) can be harvested from urine with a simple, low-cost, and noninvasive protocol. Furthermore, USCs have significant proliferation capacity and can differentiate into multiple cell lineages of the urinary wall. The differentiated USCs can express urothelial, smooth muscle, and endothelial cell markers. Also, USCs can differentiate into mesenchymal derivatives such as osteoblasts, chondrocytes, and adipocytes (9, 19).
USC has a high self-replication ability, owning a relatively long telomeres, and thus is rapid in vitro expansion. These cells maintain the chromosomal stability during in vitro culture and are safe to use for implantation without any risk of tumorigenesis (20).
The mechanisms regarding SC and tissue regeneration have not been fully understood, but it has been proven that MSCs secrete several growth factors and cytokines, exerting paracrine effects on the other cells. Because of the limited survival and differentiation of MSCs in vivo, MSCs secretome is considered as the main mechanism for tissue regeneration (21, 22).
3. Scaffolds
Scaffolds are constructs that facilitate the delivery of cells to the graft site in the body. These biomaterials provide a three-dimensional space and mechanical support for new tissue formation. It is important that the selected biomaterial be biocompatibile, biodegradable, and bioabsorbable, so that it enhances tissue regeneration without inducing the inflammatory responses (15). Also, these materials should mimic the properties of extracellular matrix and regulate several cellular events such as proliferation, differentiation, and programmed cell death (12).
There are three main categories of biomaterials or scaffolds: (1) synthetic matrices, (2) natural matrices, and (3) acellular tissue matrices.
3.1. Synthetic Matrices
Synthetic polymers such as the biodegradable polymers poly glycolic acid (PGA) and poly lactic acid-co-glycolic acid (PLGA) have been developed specially to have suitable structural and biological characteristics, which can be employed for high cellular expansion and differentiation. The most attractive characteristic of these materials is their ability to reconstruct any form of organs in three dimensions at a low cost (15, 23).
Application of a composite material consisting of a thin poly-L-lactide (PLLA) film with electrospun polycaprolactone (PCL) on top could result in high proliferation of seeded UCs (24).
3.2. Natural Matrices
Application of collagen-based scaffolds for tissue engineering has advantages over using synthetic polymers due to their favorable biocompatibility, biodagradablity, and natural essence. In addition, the degradation products exert no negative effect on the surrounding tissue. Collagen is often used alongside alginate as a natural matrix (23, 25).
3.3. Acellular Matrices
Decellularized tissue matrices are the most commonly used natural biomaterials that are obtained from autologous, allogenic or xenogenic sources. The cellular components of the harvested tissues are removed by adopting mechanical or chemical methods and leaving a natural platform for tissue formation. In other words, these materials are the tissue extracellular matrix (ECM) without cellular elements. The ECM scaffolds are usually obtained from small intestinal submucosa (SIS), bladder matrix (BAM), amniotic membrane, pericardium, and dermis from animal or human sources (26).
ECM scaffolds have wide range of applications in the various tissue regeneration, such as myocardium, skin, and urinary tract. After cells removal, the leaving scaffold composed of a mixture of functional and structural proteins establishes the ECM. It acts as a structural signaling scaffold and is able to affect multiple cellular functions such as adhesion, proliferation, and differentiation (27).
Choosing between synthetic or natural materials for developing a suitable scaffold is important for urinary tract reconstruction. The problem of the synthetic scaffolds is the lack of cytokines and ECM proteins which play important roles in mediating cellular expansion and differentiation. Natural scaffolds are weak in term of structural resistance and biochemical properties (9). Some researchers have used a combination of both types of materials to optimize the scaffold properties.
Recently, three-dimensional printing technology has been reported to be a promising candidate for tissue engineering and the creation of highly complex structures with accurate design, which can solve the problem of precise fabrication of hydrogel structures. Printable hydrogels are favorable scaffolds for 3D printing applications because they can create an optimal biocompatible template for living cells (28).
4. Tissue Engineering of Kidney
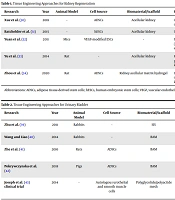
The renal tissue engineering faces some challenges due to its highly complex structure, including specialized compartments, complicated vascular network, different cell types, and multiple physiological functions. Since nephrons cannot be repaired on their own after extensive damage, choosing an appropriate scaffold with sufficient stability, porosity, and biocompatibility that is also able to enhance renal cell differentiation and nephrogenesis is necessary (29). Renal tissue reconstruction approaches are listed in Table 1.
| Research | Year | Animal Model | Cell Source | Biomaterial/Scaffold | Results |
|---|---|---|---|---|---|
| Xue et al. (30) | 2018 | - | ADSCs | Acellular kidney | Cells differentiated toward endothelial or tubular cells |
| Batchelder et al. (31) | 2015 | - | hESCs | Acellular kidney | Upregulation of renal lineage markers |
| Yuan et al. (32) | 2011 | Mice | VEGF-modified ESCs | - | Providing protection against acute kidney injury (AKI) by inducing anti-apoptotic effects and enhancing microcirculation and cell expansion |
| Yu et al. (33) | 2014 | Rat | - | Acellular kidney | Restoration of renal functions at six weeks post-operation in partially nephrectomized kidneys |
| Zhou et al. (34) | 2020 | Rat | ADSCs | Kidney acellular matrix hydrogel | Improving the survival and regenerative potential of ADSCs by acellular kidney scaffold Restoring kidney function damaged by ischemia reperfusion |
Tissue Engineering Approaches for Kidney Regeneration
Application of pluripotent SC (PSCs) such as ESCs and induced pluripotent SC (iPSCs) can substitute different renal cell types in kidney tissue reconstruction. Some studies have demonstrated that PSCs can generate metanephric mesenchyme cells giving rise to nephron progenitor cells. However, this method has not been very successful in constructing multi-functional kidneys (35).
In 2003, Poulsom et al. showed the effect of various cell type, specifically progenitor cells or SC from different sources such as bone marrow, on regeneration of acute kidney injury (36).
Adipose tissue-derived SC have the potential to differentiate into renal tubular epithelial cells (RTECs). This potential was first demonstrated by some studies in which decellularized mouse or rabbit kidney scaffolds were seeded with ADSCs through the renal artery or ureter. These experiments showed that ASCs could be embedded in the glomerular, tubular, and vascular areas of the scaffold, eventually differentiating into endothelial or tubular cells. The authors suggested that the stromal cell-derived factor 1α (SDF-1 α) enhanced cell attachment to the scaffold (30, 37). The application of acellular kidney scaffolds is a promising method for renal tissue engineering. Batchelder et al. demonstrated that using decellularized kidney scaffolds seeded with human ESC resulted in upregulation of renal lineage markers without application of any cytokine or growth factor, proposing a role for the ECM in directing renal lineage differentiation (31).
5. Tissue Engineering of Urinary Bladder
In urinary bladder tissue engineering, it should be considered that the used scaffold supports a function of adequate dynamic mechanical and chemical resistance during filling and emptying phases. Reconstructed bladder should have a compliant muscular wall and well-differentiated urothelium (38). Bladder tissue reconstruction approaches are listed in Table 2.
| Research | Year | Animal Model | Cell Source | Biomaterial/Scaffold | Results |
|---|---|---|---|---|---|
| Zhu et al. (39) | 2011 | Rabbits | - | SIS | Bladder regeneration was observed in histological andfunctional analyses. |
| Wang and Liao (40) | 2014 | Rabbits | - | BAM | Bladder regeneration was observed in histological and functional analyses |
| Zhe et al. (41) | 2016 | Rats | ADSCs | BAM | Improving the morphological regeneration of the bladder smooth muscle and nerve, and also the bladder capacity |
| Pokrywczynska et al. (42) | 2018 | Pigs | ADSCs | BAM | Constructed bladder had normal function with no sign of post-evacuation urine residual in bladders. |
| Joseph et al. (43) clinical trial | 2014 | - | Autologous urothelial and smooth muscle cells | Polyglycolide/polyactide mesh | Five patients needed further augmentation, one patient was satisfied with condition and continent, other patients showed no progression |
Tissue Engineering Approaches for Urinary Bladder
Bladder acellular matrices and SIS are commonly used in bladder tissue engineering. In two studies carried out in 2011 and 2014, the effects of BAM and SIS on bladder tissue reconstruction in the rabbit model were investigated. In both studies, similar bladder regeneration was observed in histological and functional analyses (39, 40).
Amniotic fluid derived SC (AFS), ADSCs, and SC obtained from hair have shown the potential for differentiation into various urinary bladder cells in vitro (44-46).
In another study, ADSCs obtained from adult female pigs were seeded into BAM, subsequently implanted in the pig’s bladder wall defect. Macroscopic, histological, immunofluorescence, and molecular evaluations three months after transplantation showed significant regeneration of the bladder wall. In this experiment, expression of smooth muscle cell markers (calponin, caldesmon-1) and endothelial cell markers (CD31, von Willebrand factor) was significantly higher in bladder specimens (42).
Application of biomaterials seeded with UCs and SMCs is important in the generation of an ideal bladder due to the roles of these cells in urinary barrier and mechanical support (9).
6. Tissue Engineering of Ureter and Urethra
When ureteral tissue engineering is performed, scaffolds should be easily available, impermeable to urine, and flexible; they should also have suitable properties for cell proliferation and differentiation (38).
A combination of natural and synthetic biomaterials, as hybrid scaffold, can improve scaffold’s mechanical characteristics. Hence, natural biomaterials such as collagen have been reinforced with synthetic polymers to create stronger hybrid scaffolds (47).
Tissue engineering approaches for ureter and urethra are listed in Table 3.
| Research | Year | Animal Model | Cell Source | Biomaterial/Scaffold | Results |
|---|---|---|---|---|---|
| Liao et al. (8) | 2013 | Rabbits | Bone marrow MSCs and SMCs | BAM | Multilayer urothelium was observed throughout of the lumen with significant neovascularization within the center. Organized smooth muscle bundles were seen in the histological sections. |
| Meng et al. (48) | 2015 | Rabbits | ADSCs and Bladder SMCs | Bladder submucosa matrix | The Multilayered urothelium and neovascularization were detected in the grafts. |
| de Jonge et al. (49) | 2018 | Goat | - | Collagen-I‐vicryl templates | After subcutaneous preimplantation, a single layered urothelium was observed in the entire of the lumen of ureter. Newly formed tissue consisted of connective tissue with α‐SMA expressing cells and significant neovascularization |
| Yang et al. (50) | 2004 | Rabbit | - | Urethral ECM | The epithelium completely covered the ECM at three weeks after transplantation. Also, well- organized SMCs were observed. |
| Huang et al. (51) | 2014 | Rabbit | - | BAM treated with peracetic acid | Improvement in urothelium and smooth muscle regeneration and angiogenesis in the urethral defect |
| Fu et al. (10) | 2007 | Rabbit | Foreskin epidermal cell | Tubular acellular bladder matrix | Good urethral healing with no sign of strictures |
Tissue Engineering Approaches for Ureter and Urethra
Meng et al. investigated the effects of ADSCs, and SMCs seeded into bladder submucosa matrix (BSM) on the ureteral tissue engineering in a rabbit model, and found the multilayered urothelium and neovascularization in the graft (48).
In another study, ureteral reconstruction was performed using tubular templates of tubularized vicryl meshes and type-I collagen in goats. This scaffold was first prei-implanted subcutaneously, and then was implanted in the ureteral wall defect. Results showed that using prei-implanted tissue templates, compared to direct implantation of the templates, produced more favorable outcomes. Peri-implantation procedure can produce well-vascularized tissue tubes in which the initial inflammatory response is largely controlled before functional implantation (49).
Furthermore, application of BAM and decellularized ureters has been shown to result in significant ureteral regeneration (3, 8).
In a study conducted by Yang et al., the rabbit urethral ECM was utilized to regenerate urethral segmental defects (with a length of 2 cm) in male rabbits (50).
Application of BAM treated with peracetic acid (PAA) to form three-dimensional structural constructs enhanced the urothelium, smooth muscle regeneration, and angiogenesis in the urethral defect (51). Fu et al. used the allogeneic rabbit acellular bladder submucosa seeded with foreskin epidermal cells for urethral reconstruction, and discovered that it had favorable urethral healing effect with no sign of strictures (10).
It has been found that the application of 3D printing of the poly 2-hydroxyethyl Methacrylate hydrogel in combination with natural gel network sodium alginate can repair the urethral defect (28).
7. Current Challenges and Future Direction
Nonurologic tissues such as gastrointestinal fragments are usually used to repair urinary tract, which is associated with many complications. Knowledge of bioengineering and urologic tissue has been increasing, yet it has not been put into clinical practice. Despite the significant advances toward in vitro and some animal studies, no progress has been achieved toward creation of a feasible outcome for the clinical and commercial application. One of the main challenges in this regard is the selection of an appropriate scaffold with required specifications such as biocompatibility, biodegradability, and good mechanical strength. Natural matrices provide a suitable microenvironment for cell adhesion and proliferation but possess poor mechanical features. In contrast, synthetic polymers have good mechanical strength but are not satisfactory substitutes for ECM. In this regard, bioprinting technology seems to be a promising candidate for tissue engineering.
By using cell differentiation techniques and different cell sources, researchers have regenerated the urothelial and smooth muscle cells of the urinary tract wall. Although these differentiated epithelial cells have the ability to express specific markers of urothelium, proving their ability to form a functional barrier and integration of urothelial function with neuronal signaling requires conducting further research. In addition, more investigations are needed to ensure proper alignment of the regenerated smooth muscle and sufficient neovascularization.
In case of urinary bladder, the current clinical method of tissue reconstruction has not yielded satisfactory result to replace enterocystoplasty. Although some clinical trials have found continence in a few patients, no study has reported spontaneous evacuation due to the absence of neuronal networking as seen in a native bladder (52). Despite promising advances achieved in the field of urinary tract tissue engineering, it was recommended that future studies should be carried out in order to develop proper techniques for improving angiogenesis and establishing neural connections and, therefore, creating an ideal and functional graft.
8. Conclusions
In sum, tissue engineering was a promising and effective technique for urinary tract replacement. Due to the limitations of tissue transplantation, the focus of recent investigations was mainly on tissue engineering. However, tissue engineering of hallow organs such as bladder encountered some obstacles including the ability of implanted muscles to contract, enough angiogenesis, and nerve supply. It was found that peri-implantation procedure may have created well-vascularized tissue and controlled the inflammatory response, which increased the probability of generating more effective tissues for reconstruction. Despite considerable advances achieved in tissue engineering field, several issues (e.g., the ideal cell source, suitable scaffold, and animal models) were found to remain unresolved. Therefore, it was recommended that more studies - clinical trials with larger sample sizes, in particular - should be conducted in order to confirm the amenability and usefulness of tissue engineering for urinary tissue substitution.