1. Background
Diabetes mellitus is one of the world's five leading causes of death (1). It is estimated that by 2030, the number of people with diabetes will reach more than 366 million, which is more than double the number in the year 2000 (2).
This disease is a metabolic disorder of the body caused by multiple causes, manifested by a chronic increase in blood sugar along with a disturbance in carbohydrate, fat, and protein metabolism (3). Today, diabetes mellitus is divided into two main categories: Insulin-dependent diabetes and insulin-independent diabetes. From the point of view of pathophysiological changes, both types of disease have similarities and differences (4).
This disease causes many chronic disorders, including kidney failure (nephropathy), vision (retinopathy), peripheral nervous system (neuropathy) and large blood vessels (atherosclerosis), and increased blood pressure through an excessive increase in blood glucose (5, 6).
Diabetic nephropathy is one of the most common microvascular complications of diabetes mellitus and is one of the main causes of end-stage kidney disease worldwide and is one of the factors that increase the death rate in diabetic patients (7).
Diabetic nephropathy is a progressive deterioration of kidney function characterized by changes in glomerular filtration rate (GFR), glomerular hypertrophy, and urinary albumin leakage (8). Therefore, early identification of the risk of diabetes and its microvascular complications can be an opportunity for preventive interventions to prevent or delay the disease, which is important to reduce the morbidity and mortality of type 2 diabetic patients (8).
Many biomarkers play an important role in the diagnosis and prognosis of diabetic nephropathy (9).
An increase in thyroid-stimulating hormone (TSH) and a decrease in T3 are usually observed in diabetic patients (10).
It has been reported that a high TSH level is associated with the progression of kidney disease and the development of malignancy, and on the other hand, a low T3 level is associated with inflammation in chronic kidney disease patients (11, 12).
Several studies have also observed that type 2 diabetic patients with subclinical hypothyroidism are associated with a high prevalence of diabetic nephropathy (12, 13).
2. Objectives
The aim of this study is to investigate the effect of thyroid hormones on diabetic nephropathy in type 2 diabetic patients referred to Ahvaz hospitals in 2017. If there is a positive correlation between thyroid hormones and diabetic nephropathy, these hormones can be considered biomarkers for predicting diabetic nephropathy and preventing it in type 2 diabetic patients.
3. Methods
In this case-control study, 100 people with type 2 diabetes with complications of diabetic nephropathy were taken as the case group and 100 people with type 2 diabetes as the control group with a consent form. Both groups had type 2 diabetes based on the criteria of the American Diabetes Association (ADA).
Type 2 diabetes criteria, according to the American Diabetes Association, are having fasting plasma sugar above 126 mg/dL with two repetitions and typical symptoms of diabetes such as binge drinking or having plasma glucose above 200 mg/dL based on the oral glucose tolerance test with 75 grams of glucose orally (OGTT) or random plasma glucose above 200 mg/dL with typical symptoms of diabetes or hemoglobin A1C ≥ 6.5% (14).
In this research, the criteria for entering the test for type 2 diabetes are complete consent and awareness, no heart failure based on examination and history, no adrenal insufficiency, Cushing's syndrome and hypothyroidism based on examination and history, and no use of painkillers for any reason. And the exclusion criteria are chronic diseases such as liver diseases, kidney diseases, stroke, infectious diseases, cancer, allergies, and diseases that are based on immunity, use of immunosuppressant drugs, statins, and thiazolidinedione family, and It reduces blood fat and blood diseases.
Considering that serum was needed to perform the tests, about 3 mL of blood was taken from all people in a tube without an anticoagulant, and after the blood was clotted, it was centrifuged at 4 degrees Celsius for a while. Centrifugation was performed for 20 minutes with a force of 936 g (equivalent to 3000 rpm). After centrifugation, the serum was divided into several microtubes using a sampler and stored in a -70°C freezer.
3.1. Biochemical Assays
Using isolated serum, fasting blood glucose, BUN, uric acid, creatinine, calcium, and phosphorus were measured using the kits of Pars Azmoun Company based on enzyme principles. These measurements were measured using a Hitachi Autoanalyzer (Hitachi 2017, Model 7180). In all the above tests, quality control principles were followed in accordance with national and international standards
3.2. Hormone Assay
The serum level of thyroid hormones (T3 and T4) and the serum level of TSH were measured according to the instructions of the ARCHITECT kit using the immunoluminescence quantitative method and a quantitative luminescence device (Abbot ARCHITECT i2000 plus). In all the above experiments, the principles of control quality were observed in accordance with national and international standards.
3.3. Data Analysis Method
The data obtained from the qualitative variables were analyzed using the chi-square test. Quantitative variables were expressed as mean ± standard deviation. In order to choose the appropriate statistical test, first, the normality of the data and the equality of variances were checked with the Kolmogorov-Smirnov method. Data with normal distribution were compared using Student's t-test, and the Mann-Whitney U test was used to compare data with non-normal distribution.
Spearman's correlation analysis was performed to identify the relationship between the levels of thyroid hormones and serum TSH hormone with other variables. The relationship between thyroid hormones and TSH in diabetic nephropathy was evaluated using logistic regression. Examining the diagnostic value of these hormones was also done with receiver operating characteristic (ROC) curve analysis. Statistical analyses were performed using SPSS (Chicago, IL) version 21. P < 0.05 was considered a significant level.
4. Results
4.1. Anthropometric and Laboratory Characteristics of the Studied Population
The study population included 100 diabetics as a control group and 100 diabetics with nephropathy complications as a test group. Information related to the demographic and laboratory characteristics of the studied people is shown in Table 1.
| Variables | Control Group | Case Group | P-Value |
|---|---|---|---|
| Age (y) | 60.6 ± 7.9 | 61.6 ± 7.7 | 0.461 |
| Sex (male), n | 56 | 59 | 0.668 |
| FBS | 190.8 ± 57.5 | 186.2 ± 63.1 | 0.195 |
| BUN | 17.3 ± 5.7 | 62.1 ± 18.9 | < 0.001 |
| Creatinine | 0.9 ± 0.3 | 6.9 ± 2.2 | < 0.001 |
| UA | 5.1 ± 1.3 | 7.1 ± 1.1 | < 0.001 |
| Ca | 9.2 ± 0.8 | 8.4 ± 0.7 | < 0.001 |
| Phos | 4.3 ± 1.0 | 5.5 ± 1.5 | < 0.001 |
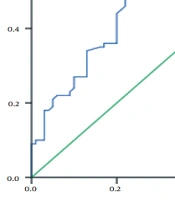
| TSH (mIU/L) | 1.7 ± 1.2 | 3.1 ± 3.3 | < 0.001 |
| T3 (ng/mL) | 0.86 ± 0.15 | 0.57 ± 0.17 | < 0.001 |
| T4 (μg/dL) | 7.1 ± 0.9 | 5.7 ± 1.5 | < 0.001 |
a Values are expressed as mean ± SD.
According to Table 1, the average age, gender, and sugar levels of both groups of participants in the experiment were not statistically significantly different.
However, a significant difference was observed in BUN, creatinine, and uric acid, biomarkers for kidney function (P < 0.001). On the other hand, the amount of calcium and phosphorus between the control group and the patients was significantly different (P < 0.001).
Figure 1 shows the levels of TSH, T4, and T3 hormones in patients with diabetic nephropathy compared to diabetic patients without nephropathy complications. In contrast, the TSH hormone in the diabetic nephropathy group had a higher concentration than in control. After statistical analysis, it was observed that there was a significant difference between the two groups (P < 0.001).
Table 2 shows the correlation analysis between the investigated variables and thyroid hormones and TSH in the studied population.
| Variables | TSH | T3 | T4 | |||
|---|---|---|---|---|---|---|
| r | P-Value | r | P-Value | r | P-Value | |
| Age | 0.067 | 0.346 | -0.099 | 0.162 | -0.047 | 0.506 |
| FBS | -0.101 | 0.156 | 0.081 | 0.253 | 0.056 | 0.434 |
| BUN | 0.219 a | 0.002 | -0.607 a | < 0.001 | -0.480 a | < 0.001 |
| Creatinine | 0.200 a | 0.005 | -0.598 a | < 0.001 | -0.433 a | < 0.001 |
| UA | 0.173 a | 0.015 | -0.434 a | < 0.001 | -0.301 a | < 0.001 |
| Ca | -0.086 a | 0.228 | 0.334 a | < 0.001 | 0.351 a | < 0.001 |
| Phos | 0.070 | 0.332 | -0.188 a | 0.008 | -0.173 b | 0.014 |
| TSH | 1.000 | - | 0.177 b | 0.012 | 0.237 a | 0.001 |
| T3 | -0.188 a | 0.008 | 1.000 | - | 0.524 a | < 0.001 |
| T4 | -0.129 | 0.069 | 0.524 a | < 0.001 | 1.000 | - |
a Correlation is significant at the 0.001 level.
b The correlation is significant at the 0.05 level.
As can be seen in the table below, a positive and significant correlation was observed between T3 and T4 hormones (P < 0.001, r = 0.524) and TSH hormone negatively and significantly with T3 hormone (P < 0.008, r = -0.188) was related.
4.2. Correlation Analysis Between the Variables of The Studied Subjects
In addition, a positive and significant relationship was obtained between thyroid hormones and calcium. At the same time, a negative and significant relationship was observed between thyroid hormones and phosphorus. On the other hand, TSH hormones are positively and significantly correlated with kidney evaluation markers such as creatinine (P < 0.005, r = 0.200), uric acid (P < 0.015, r = 0.173), and BUN (P < 0.002, r = 0.219) was related.
4.3. Relationship Between Thyroid Hormones and TSH Hormones in Diabetic Nephropathy
The relationship between thyroid hormones and TSH hormones in diabetic nephropathy was investigated using logistic regression. Table 3 shows the odds ratio of the effect of thyroid hormones and TSH hormones on diabetic nephropathy. Considering the possible effect of age and gender on the relationship between thyroid hormones and TSH hormones on diabetic nephropathy, this relationship was adjusted for these possible confounding factors. The results showed that increased TSH and decreased thyroid hormones could increase the risk of developing diabetic nephropathy. Meanwhile, the T3 hormone is strongly related to diabetic nephropathy (0.000002 - 0.001057) (CI) = 0.000048 = Odds ratio. Considering the possible effect of age and gender on the relationship between thyroid hormones and TSH hormone in diagnosed idiopathic, this relationship was adjusted for these possible confounding factors.
| Variables | Odd Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Age | 1.002 | 0.953 - 1.054 | 0.764 |
| Sex | 0.778 | 0.337 - 1.801 | 0.474 |
| TSH | 1.392 | 1.100 - 1.762 | 0.006 |
| T4 | 0.674 | 0.489 - 0.930 | 0.016 |
| T3 | 0.000059 | 0.000003 - 0.001268 | < 0.001 |
4.4. Diagnostic Value of Thyroid Hormones and TSH Hormone Levels for the Differentiation of Nephropathy in Diabetic Patients
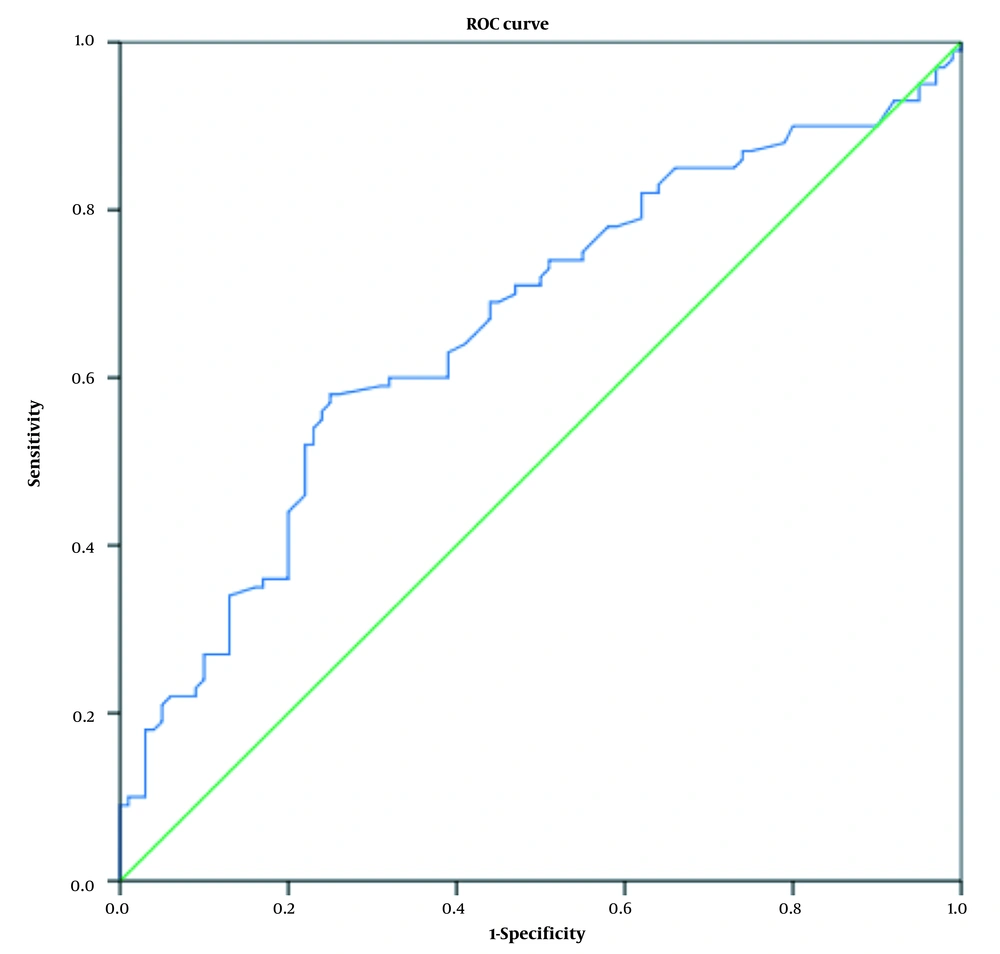
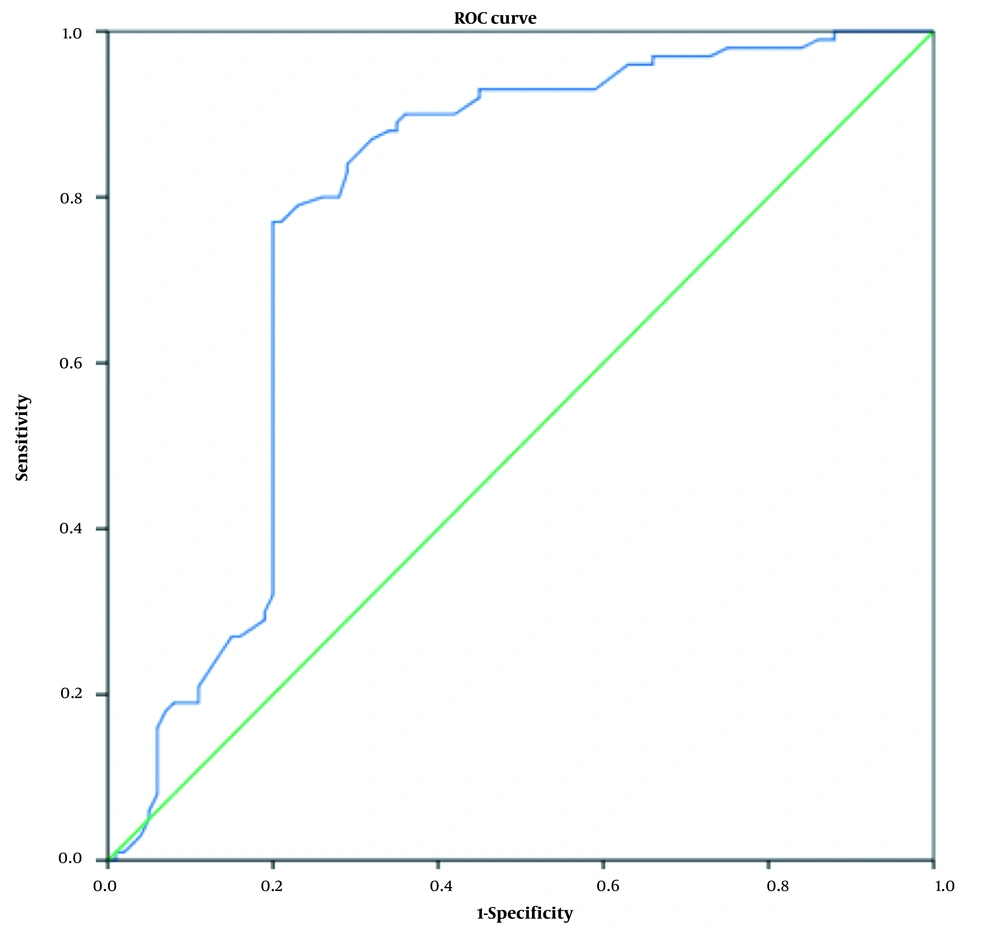
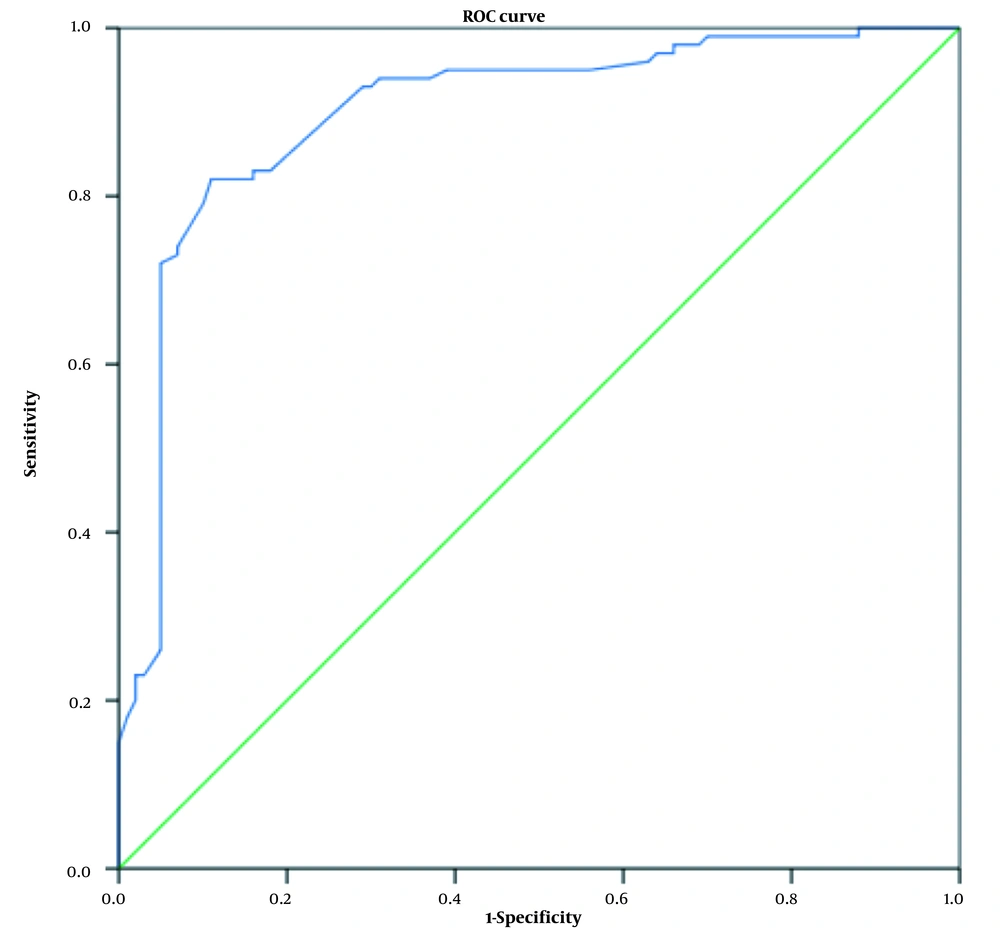
According to the relationship between thyroid hormones and TSH hormone with diabetic nephropathy using logistic regression, the diagnostic value of these hormones was also investigated. For this purpose, a ROC curve analysis was performed. Figures 2 - 4 show the ROC curve for examining the diagnostic value of thyroid hormones and TSH hormone in the differentiation of nephropathy in diabetic patients. Receiver operating characteristic curves were used to determine the accuracy of the diagnostic value of thyroid hormones and TSH for the differentiation of nephropathy in diabetic patients and their optimal cut-off values for the research group. The area under the ROC curve (AUC) was calculated to evaluate the diagnostic value of thyroid hormones in the differentiation of nephropathy in diabetic patients.
Table 4 also shows the diagnostic value of these hormones in differentiating nephropathy in diabetic patients and the sensitivity and specificity of these biomarkers. The results showed that the TSH hormone has a low diagnostic value compared to thyroid hormones, and this hormone at the level of 1.72 mIU/L with a sensitivity of 63% and a specificity of 61% can be considered a marker for the differentiation of diabetic nephropathy (Figure 2).
| Variables | Area | 95% Confidence Interval | Cut-off Values | Sensitivity (%) | Specificity (%) | P-Value |
|---|---|---|---|---|---|---|
| TSH | 0.663 | 0.587 - 0.738 | 1.72 mIU/L | 63 | 61 | < 0.001 |
| T4 | 0.780 | 0.711 - 0.849 | 6.75 μg/dL | 80 | 74 | < 0.001 |
| T3 | 0.899 | 0.854 - 0.945 | 0.73 ng/mL | 83 | 84 | < 0.001 |
T4 hormone at 6.75 μg/dL has a diagnostic value with 80% sensitivity and 74% specificity for distinguishing nephropathy in diabetic patients (Figure 3).
On the other hand, the T3 hormone has a high diagnostic value, and at levels of 0.73 ng/mL, it has a diagnostic value with 83% sensitivity and 84% specificity for distinguishing nephropathy in diabetic patients (Figure 4).
5. Discussion
Diabetic nephropathy is one of the most common microvascular complications of diabetes mellitus and is one of the main causes of end-stage kidney disease worldwide and is one of the factors that increase the death rate in diabetic patients (15).
The results of the present study showed that thyroid hormones were significantly lower in the group of patients with diabetic nephropathy compared to diabetic patients without nephropathy complications. In comparison, the TSH hormone in the diabetic nephropathy group had a higher concentration than in the control. Also, thyroid hormones were negatively and significantly correlated with kidney assessment markers such as creatinine, uric acid, and BUN. In contrast, the TSH hormone was positively and significantly correlated with kidney assessment markers.
Other results of this study showed that the increase in TSH hormone and the decrease in thyroid hormones could increase the chance of diabetic nephropathy. This is even though the T3 hormone is strongly related to diabetic nephropathy and has a high diagnostic value as a biomarker for the differentiation of diabetic nephropathy, but the TSH hormone has a lower diagnostic value compared to thyroid hormones.
Consistent with our study, in 2018, Fei et al. evaluated thyroid hormones in 301 type 2 diabetic patients. They observed that patients with nephropathy had lower T3 and free T3 (FT3) levels than patients without nephropathy, but TSH levels were increased in patients with nephropathy (8).
A similar study was conducted in the Iranian population in 2017 by Mansournia et al., who studied 255 patients with type 2 diabetes (without any history of thyroid hormone disorders). They showed that subclinical hypothyroidism and diabetic nephropathy were 18.1% and 41.2%, respectively, among the participants. It was also observed that subclinical hypothyroidism is independently associated with a high rate of diabetic nephropathy. The interesting point was that according to their report, the prevalence of subclinical hypothyroidism in Iranian diabetic patients has the highest prevalence reported in the world (16).
In contrast to our results, in 2013, Rai et al. investigated thyroid hormone levels in diabetic patients with and without diabetic nephropathy. They showed no correlation between T3, T4, and TSH with serum creatinine and glycated hemoglobin in patients with type 2 diabetes without complications and with complications of diabetic nephropathy. But they reported a significant relationship between T3 and urinary microalbumin in patients with diabetic nephropathy (17).
5.1. Conclusions
The results of this study showed that the increase in TSH hormone and the decrease in thyroid hormones could increase the chance of developing diabetic nephropathy. Meanwhile, the T3 hormone is strongly associated with diabetic nephropathy. So the T3 hormone has a high diagnostic value, and at levels of 0.73 ng/mL, it has a diagnostic value with 83% sensitivity and 84% specificity for the differentiation of nephropathy in diabetic patients. Also, the T4 hormone at 6.75 μg/dL has a diagnostic value with 80% sensitivity and 74% specificity for the differentiation of nephropathy in diabetic patients.