1. Introduction
Acute respiratory syndrome (SARS)-coronavirus (CoV)-2 causes coronavirus disease (COVID-19). It presents primarily as an acute respiratory illness with interstitial and alveolar pneumonia but can affect multiple organs, including the kidneys, heart, gastrointestinal tract, blood, and nervous system. The rapidly spreading flare-up started in Wuhan, Hubei Area, China, in December 2019 and was then declared a pandemic (1). The most common clinical indications of COVID-19 were fever (98%), cough (76%), myalgia, and fatigue (18%), accompanied by leukopenia (25%) and leukopenia (63 %). Signs of upper respiratory tract infection with runny nose and hacking are rare except in children. About 20% of the patients present with serious symptoms, and about 16 - 20% of them have acute and distressing symptoms (2). Initial reports suggest a lower incidence of ARI (3% to 9%) in people infected with COVID-19 (3, 4). According to the studies on 59 patients with COVID-19, about one-third of the admitted patients showed albuminuria on the first day, and about 53% had protein excretion symptoms during their hospital stay (5). According to the results of the blood tests, about 27% of the patients experience an increase in their blood urea nitrogen level, and this increase is also detectable in the blood of two-thirds of the deceased people.
On the other hand, computer tomography has shown a decrease in the density of the kidneys, which could be due to inflammation. All these studies have highlighted the importance of acute kidney injury (AKI) in the hospital mortality of patients with COVID-19 (6).
2. Case Presentation
A 37-year-old female patient with two positive COVID PCR and Cr = 0.8 but with no history of AKI was admitted to Razi Hospital in Ahvaz in April 2020 for ten days. She was treated with naproxen and hydroxychloroquine and then was discharged when the symptoms of fever and dyspnea were resolved and the COVID PCR test result was determined negative.
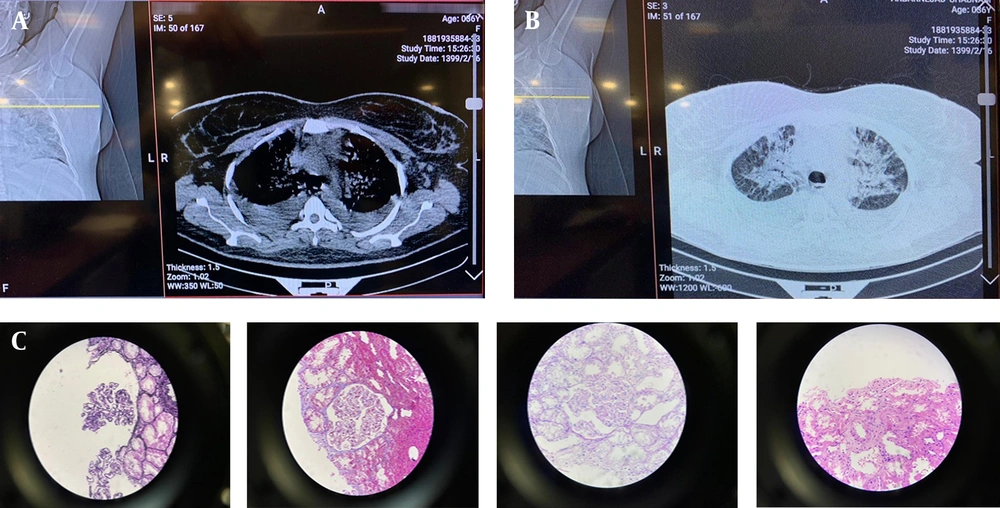
On April 23, 2020, the patient presented with symptoms of weakness, lethargy, and edema of the lower extremities; therefore, creatinine analyses were performed after the COVID-PCR test returned negative. The patient’s echocardiography showed EF = 50 - 55% and mild PE (Figure 1A).
A, Echocardiography. On April 23, 2020, when the patient was admitted to Golestan Hospital for the second time, her echocardiography showed EF = 50 - 55% and mild PE; B, Chest CT. Chest CT showed pleural congestion in the patient; C, Results of kidney biopsy. The results of the renal biopsy showed that the histological findings were consistent with thrombotic microangiopathy.
She was admitted to Golestan Hospital with a high creatinine level and acute renal injury. The result of the patient’s echocardiography was the same. From April 27 to 29, she was given two doses of prednisolone (1 g). A renal biopsy and other tests were performed on her. The results of the second test were normal (Table 1).
| Test | Result | Unit | Normal Range |
|---|---|---|---|
| Rheumatoid | |||
| Anti-GBM Ab | Negative | Titer | Up to 1.10 |
| Anti SSA (Ro) Ab | Negative (0.2) | Ru/mL | Negative < 20; positive ≥ 20 |
| Anti SSB (La) Ab | Negative (0.8) | Ru/mL | Negative < 20; positive ≥ 20 |
| Anti dsDNA Ab | Negative (1.1) | IU/mL | Negative < 30; grey zone: 30 - 35; positive > 35 |
| ANCA (P) | Negative | Index | Elisa: negative < 1.0; positive ≥ 1.0; if: negative < 1.10; positive > 1.10 |
| ANCA (C) | Negative | Index | Elisa: negative < 1.0; positive ≥ 1.0; if: negative < 1.10; positive > 1.10 |
| Anti-CCP Ab (CPA) | Negative (0.8) | U/mL | Negative < 30; positive ≥ 30 |
| Anti-phospholipid Ab (IgG) | Negative | U/mL | Negative < 10; positive ≥ 10 |
| Anti-phospholipid Ab (IgM) | Negative | U/mL | Negative < 10; positive ≥ 10 |
| Anti-cardiolipin Ab (IgG) | Negative | U/mL | Negative < 10; positive ≥ 10 |
| Anti-cardiolipin Ab (IgM) | Negative | U/mL | Negative < 10; positive ≥ 10 |
| Coagulation | |||
| Lupus anticoagulant | 37.4 | Sec | 31 - 45 |
| DRVV screen | 1.3 | Sec | Normal: absence < 1.2; abnormal: presence > 1.2 |
| Special biochemistry | |||
| C3 | 1.24 | g/L | 0.9-1.8 |
| C4 | 0.256 | g/L | 0.09-0.39 |
| CH50 | 60.0 | % | Low < 50; normal 50-150; high > 150 |
| Serology | |||
| RF | Negative | IU/mL | Quantitative (IU/mL) up to 20; qualitative: normal: negative |
Four days later, the patient was discharged from the hospital and was given a prescription including Nephrovit, folic acid, erythropoietin, prednisolone 75 mg, and allopurinol.
The next day, our patient returned to the hospital with dyspnea and productive cough symptoms. She had a fever (37.8), oxygen saturation of 80%, abdominal distention and ascites, three plus-limb edema (anasarca edema), and fine crackles (rales) at the top of her both lungs. TAP Ascitic fluid was examined in the patient (Table 2). The results of the patient’s body fluid (ascites) examination showed a high protein content and a high SAAG value. Following the advice about treating pulmonary problems caused by a coronavirus, the patient was transferred to the isolation ward of the intensive care unit and was prescribed meropenem at a modified dose, vancomycin at a modified dose, furosemide, serum TNG (nitroglycerin), prednisolone (50 mg).
| Liquid Test | Result | Unit | Normal Range |
|---|---|---|---|
| Appearance | Yellow, semi-clear | Clear | |
| Total cell count | 68 | ||
| RBC | 33 | ||
| Leukocytes | 35 | ||
| Neutrophils | 15 | % | |
| Lymphocytes | 20 | % | |
| Glucose | 129 | Mg/dL | 50 - 80 |
| Protein | 3.5 | Mg/dL | 15 - 45 |
| Albumin | 2.5 | ||
| LDH | 139 | ||
| Other | * |
a The results of the patient’s body fluid (ascites) examination showed a high protein content and a high SAAG value.
Following the advice of the nephrologist (suspected PCP), our patient was treated with prednisolone and co-trimoxazole injections. The chest CT showed pleural congestion in the patient (Figure 1B).
The next morning, the patient was prescribed IVIG; however, she presented with decreased O2sat before receiving IVIG, resulting in respiratory dystrophy. Her COVID PCR test was positive, and, therefore, she was intubated; but she died despite all treatments.
The results of the renal biopsy showed that the histological findings were consistent with thrombotic microangiopathy (Figure 1C).
A maximum of 27 glomeruli were seen in the examined serial sections. The glomeruli showed mild enlargement with simplification of the tuft architecture due to the segmental to global mesangial expansion with a fluffy and hyaline appearance (mesangiolysis). Mesangial cells were not proliferated. Glomerular capillaries are usually devoid of erythrocytes and show narrowing of the lumen due to endothelial swelling. Rare fragmented erythrocytes or fibrin thrombi are seen in some capillaries and arterioles of the hilus. The capillary walls showed irregularities and wavy contours.
As for the tubules, degenerative vacuolization of the epithelial cells of the renal tubules was seen in some of the proximal tubule profiles.
3. Discussion
COVID-19, which was first reported in one of the cities in China, killed hundreds of thousands of people in a very short period. Acute kidney injury is one of the symptoms of this disease, which affects 0.5 to 7 percent of the patients and results in the admission of 2.9 to 23 percent of them to hospitals and ICUs (7, 8).
Wang et al. (9) showed that AKI was uncommon in COVID-19. SARS-CoV-2 infection does not cause AKI or exacerbate CKD in COVID-19 patients. In our case report, the patient had no previous history of AKI; however, she developed AKI as a result of the COVID-19 infection.
At the time of our report, it was not known how the kidneys were involved in this disease, but the sepsis caused by the virus may have led to direct cell damage and cytokine storm. SARS-CoV and MERS-CoV bind to, respectively, angiotensin-converting enzyme (ACE) and dipeptidyl peptidase-4, which are expressed in renal tubule cells (10, 11). In both infections, viral RNA was detected in renal tissue and urine (12). Recent studies conducted at the Zhong laboratory in Guangzhou showed that they were able to isolate the SARS-CoV-2 virus from the urine samples of the infected people. This study showed that the kidney was probably one of the main targets of this virus (13). In the context of SARS-CoV infection, it has been found that the interaction between the spike protein (S protein) of SARS-CoV-2 and the angiotensin-converting enzyme 2 (ACE2) receptor present in host cells is crucial for viral entry. Activation and cleavage of the S protein take place via cellular transmembrane serine proteases (TMPRSS), thereby producing fusion peptides that facilitate membrane fusion and subsequent viral release (14, 15). The co-expression of ACE2 and TMPRSS2 plays a vital role in facilitating the entry of SARS-CoV-2 into the host cells, thereby intensifying the host conditions conducive to the proliferation of the coronavirus.
Pan et al. unequivocally identified the host cells of the kidney as podocytes and proximal straight tubule cells (15). The podocytes and proximal straight tubule cells assume a crucial function in the processes of urine filtration, reabsorption, and excretion. Podocytes exhibit a heightened vulnerability to viral and bacterial infections, and their damage invariably leads to the onset of substantial proteinuria (16). The present study postulated that the pathogenesis of AKI in individuals suffering from COVID-19 may have been attributed to the cytopathic effects of SARS-CoV-2 on podocytes and proximal straight tubule cells, particularly in those who demonstrate SARS-CoV-2 infection within their blood samples. Therefore, it is imperative to prioritize diligent early renal function monitoring and cautious management of the urine of COVID-19-afflicted individuals with AKI in order to avert inadvertent transmission of the disease. In this study, a case of COVID-19 with AKI was reported. Overall, patients with a high prevalence of concomitant diseases such as AKI may have suffered from severe forms of COVID-19. Therefore, it was recommended that the risk of AKI, in addition to the risk of respiratory failure, should be considered in patients with COVID-19. Furthermore, COVID-19 may have shown symptoms of AKI in patients without a history of such a condition.
3.1. Conclusions
It was concluded that the patients with a high prevalence of comorbidities such as AKI may have suffered from severe forms of COVID-19. In patients with COVID-19, therefore, it was recommended that the risk of AKI should be considered in addition to the risk of respiratory failure. Moreover, COVID-19 may have presented symptoms of AKI, even in patients with no history of this condition.