1. Background
Colorectal cancer develops in the colon or the rectum. The colon is known as the "large intestine," and the rectum is at its end (1). Colon cancer is a malignant gastrointestinal cancer, the second most common after breast cancer in women and the third most common in men after lung and prostate cancer (2). Polyps are groups of cells that grow in the colon mucosa and may turn into malignant tumors (3). Nutrition is one of the most important and preventable risk factors for colorectal cancer, so 30 - 50% of its cases can be prevented with proper nutrition (4).
In recent years, medicinal plants have been used to prevent and treat all types of cancer due to their cheapness and fewer effects. One of these plants is curcumin, which is a polyphenolic compound obtained from the plant Curcuma longa (turmeric spice), which is part of the ginger family. Studies have shown that curcumin regulates several molecules in colon cancer signaling pathways (5). Curcumin treats and disinfects wounds in East Asian countries (6). Studies show that curcumin has many effects, including anti-inflammatory, antioxidant, antibacterial, blood anticoagulant, etc. Recently, its anticancer properties have been shown in breast, liver, lung, skin, colon, and pancreatic cancer (7).
The emerging method of photodynamic therapy destroys damaged cells, such as cancer cells or cells infected with microorganisms and unwanted tissues. The working principle of photodynamics is based on stimulating a suitable sensitizer with characteristics such as non-toxicity, selective reaction, and retention by the tumor tissue and creating oxygen-free radicals by absorbing wavelengths that can easily pass through the tissue. The photosensitizer can react with the surrounding molecular oxygen in two ways (8). Curcumin, which is a polyphenolic compound, is chosen as a photosensitizer. This photosensor is herbal and has no side effects (6).
The nanoparticles that carry these photosensitizers are magnetic iron oxide nanoparticles, which by coating them with silica, make this material non-toxic (9).
The light absorption of curcumin is at 450 nm wavelength, so a 450 nm blue laser whose intensity is reduced to 150 mW by an optical lens is used (10).
This amount of wavelength causes absorption by curcumin and leads to the transfer of magnetic nanoparticles to the site of cancer cells and the release of reactive oxygen species at the tumor site, and the killing of cancer cells (11). The studies of Garcea and his colleagues in 2005 showed that in prescribing curcumin capsules of 4500, 1800, and 3600 mg daily to patients with colon cancer for 7 days, the dose of 3600 mg of curcumin is pharmacologically effective (12).
On the other hand, He and his colleagues in 2011 studied 126 patients with colon cancer before surgery and prescribed curcumin at a dose of 360 mg (3 times a day) and compared it with the placebo group, and concluded that curcumin decreased serum TNF-α level, modulated tumor cell apoptosis and increased P53 expression. Also, the general health level of the patients had improved (13).
2. Objectives
This research aims to investigate the effects of curcumin and low-power laser therapy on colon cancer cells. We hope that the results of this study and the photodynamic therapy method will help destroy colon cancer cells and treat this disease faster.
3. Methods
This research was conducted in 2015 - 2020 at the Afra Biology Laboratory (Tehran).
3.1. Cell Culture
Colorectal adenocarcinoma cell line (Caco2) was obtained from the Pasteur Institute cell bank and cultured in a culture medium containing 90% DMEM and 10% fetal bovine serum. The cells were kept in standard conditions of 37°C and 5% of carbon dioxide, and about 80% humidity. The cell culture medium was changed every two days.
3.2. Study of Curcumin
Based on previous studies, logarithmic concentrations of 10, 50, 100, 500, and 1000 micrograms per milliliter of curcumin (Sigma) were selected. The MTT test performs curcumin's cytotoxicity, a colorimetric method based on reducing and breaking yellow tetrazolium (MTT) crystals (MELFORD, manufactured in Etgolestan) by succinate dehydrogenase enzyme and, finally, the formation of insoluble blue crystals was evaluated (14).
For this purpose, cells were cultured with an optimized number of 10,000 in each well of a 96-well plate with a final volume of 100 microliters of specific culture medium, then specific concentrations of curcumin were added to each well. Finally, the absorbance at 570 nm wavelength was read by a plate reader (BioTeK, USA) for each well. This experiment was repeated in triplicate.
Finally, the level of cytotoxicity was obtained using the following relationship. This relationship shows the survival percentage of cells, and by subtracting it from 100%, the toxicity of cells was determined as a statistical average of three independent experiments.
Vital cell percentage = (drug - treated photo absorbed cells - absorbed optical blank) / (absorbed optical control - absorbed optical blank) × 100
Investigating the effect of low-power laser: First, the cell suspension prepared from Caco2 colorectal adenocarcinoma cancer cells containing 1 × 104 cells were cultured in 96-well plates. Twenty-four hours after cultivation, the cells were exposed to laser radiation (Pioon model laser with a wavelength of 1470 - 450 nm) with a wavelength of 470 nm for 90 seconds.
The cells were placed in an incubator for 24 hours at a temperature of 37°C and an atmosphere of 5% CO2. After 24 hours, 20 microliters of MTT were added to each well. They were kept in the incubator for 4 hours, and 100 microliters of DMSO were added to each of the wells to dissolve the resulting formazan. After 30 minutes, an ELISA reader read the absorbance of formazan in the absorbance spectrum of 570, and the survival rate was calculated according to the relevant formula.
Low-power laser combined with curcumin: In this step, the cell suspension prepared from Caco2 colorectal adenocarcinoma cells containing 1 × 104 cells was cultured in 96-well plates. Twenty-four hours after cultivation, the cells were exposed to Pioon laser radiation with a wavelength of 470 nm for 90 seconds.
Then the cells were placed in the incubator with specified amounts of curcumin for 24 hours at 37 degrees and 5% CO2 atmosphere. After 24 hours, 20 microliters of MTT were added to each well, and after 4 hours of incubation, 100 microliters of DMSO were added to each well to dissolve the formazan. After 30 minutes, the absorbance of formazan was read by an ELISA reader (Stat Fax-2100 model, Spain) in the absorbance spectrum of 570, and the survival rate was calculated according to the relevant formula.
3.3. Colony Formation Test
The clonogenic or colony formation test is an in vitro cell viability assay based on the ability of a single cell to grow into a colony. A colony is defined as at least 50 cells. This assay tests each cell in the population for its ability to undergo "unlimited" division.
The clonogenic method is the method of choice for determining cell death after treatment with ionizing radiation, but it can also be used to determine the effectiveness of other cytotoxic agents. Only a fraction of the cells maintain the colony production capacity. Before or after treatment, cells were cultured at appropriate dilutions to form colonies for 14 days. Add 0.5 mL of cold 70% ethanol to each well to fix the colonies and incubate at 37°C for 20 - 30 minutes. Crystal violet is used for staining, and the colonies are counted under the microscope.
4. Results
The colorectal adenocarcinoma Caco2 cancer cell line had good proliferation. The proliferation rate and morphology of these cells before and after freezing were completely the same, and during the different stages of the work, no signs of contamination of these cells with viruses or mycoplasma were observed.
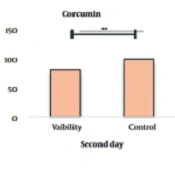
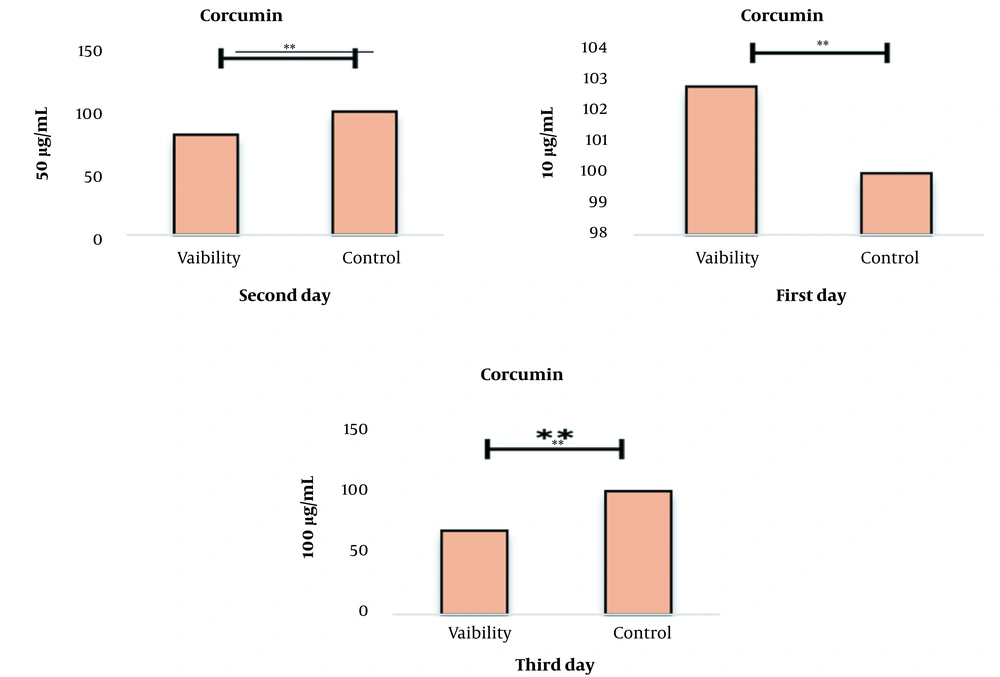
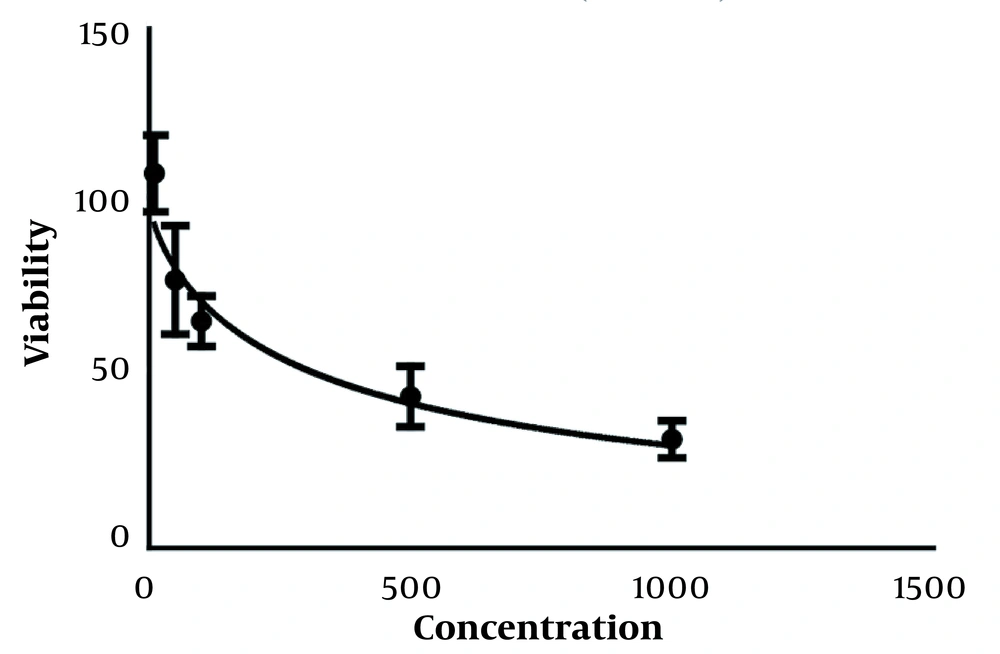
The results of the effect of curcumin on the survival rate of cancer cells using the MTT test after treatment with concentrations of 10 to 1000 µg/mL of curcumin showed that this drug could increase the percentage of living cells in different concentrations compared to the control group in a concentration and time-dependent manner (Figure 1). As shown in this graph and the following graphs, one group is considered as control, which contains the highest concentration of curcumin 0.01%.
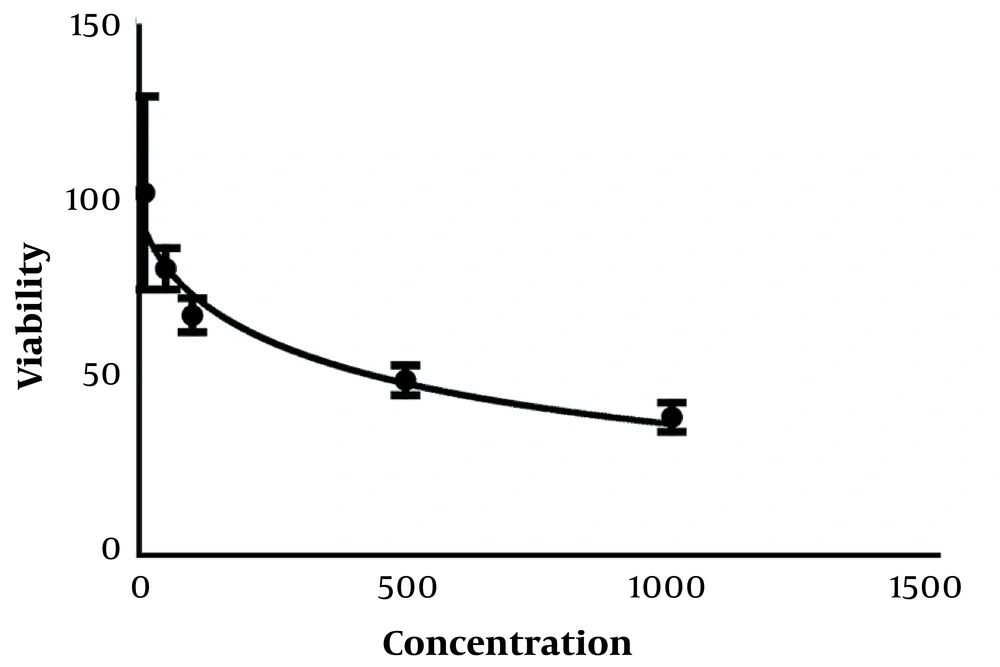
This decrease in the percentage of live cells in all concentrations compared to the control sample was significant at the P < 0.001 level. IC50 treatments for 24 and 48 hours were obtained as mL/2µg and mL/µg, respectively. Results. IC50 for 24, 48, and 72-hour treatments are given in Figure 2.
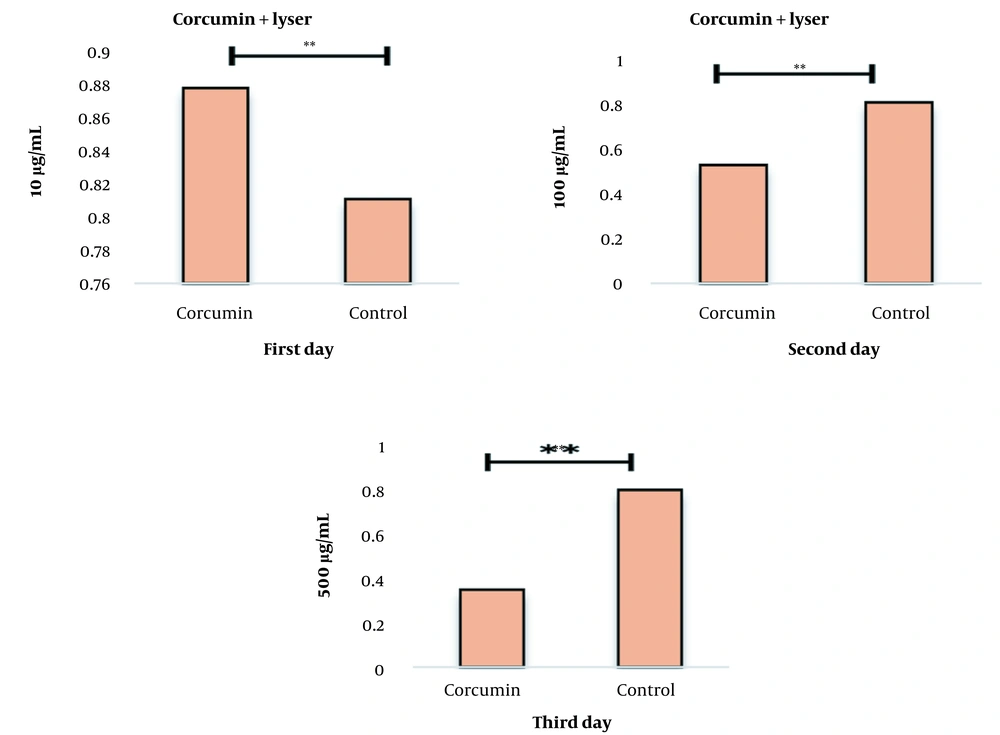
After determining the non-lethal and lethal doses, the effect of the combination of curcumin and laser was studied simultaneously on the cell survival rate. The percent of cell survival after treatment with concentrations of 10 and 50 µg/mL curcumin along with laser for 24, 48, and 72 hours are given in Figure 3. The results show that adding curcumin increases the lethal effects of laser.
Measuring the survival percentage of cells after curcumin and laser treatment using the MTT test. After 24, 72, and 48 hours, this graph shows that curcumin affects cell proliferation in a concentration-dependent manner. The results show that the combination of curcumin and laser together increases the lethal effects (P-value < 0.001).
IC50 results were obtained for 24, 48, and 72 hours treatments, and the results of examining the amount of absorption of curcumin and curcumin combined with laser and their effect on cancer cells can be seen in Figure 4. The amount of absorption of curcumin increased compared to the control group, and this amount of absorption increased more when curcumin was used together with a laser, which shows the extraordinary effect of curcumin and curcumin combined with a laser (Table 1).
| CTRL | Curcumin | Curcumin+L | |
|---|---|---|---|
| Absorbance | 0.409 | 0.84 | 0.96 |
| 0.415 | 0.76 | 0.783 | |
| 0.394 | 0.662 | 0.664 | |
| 0.728 | 0.572 | 0.712 |
The Effect of the Combination of Curcumin and Curcumin Combined with Laser on the Human Colorectal Cancer Cell Line
5. Discussion
In the present study, the effect of curcumin alone and also with low-power laser on colon cancer cells was evaluated. In this experiment, compared to normal colon mucosa cells, colorectal cancer cells were exposed to drugs and lasers for 24, 48, and 72 hours by the MTT test. The selection of curcumin concentrations was based on previous reports from previous studies.
Clonogenic and MTT tests were used to calculate cell survival after laser beam irradiation, and the results show the concordance of the survival curves obtained from MTT and clonogenic tests. The anticancer properties of curcumin, also known as diferuloylmethane [1,7-bis- (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], have been extensively studied. Studies show that curcumin inhibits carcinogenesis in clinical trials on various cell lines, including leukemia, prostate, pancreatic, ovarian, oral epithelial, liver, breast, cervical, gastric, and colon cancers (14-16).
A study by Han and his colleagues in 2012 showed that curcumin inhibits protein phosphatases 2A and 5, which leads to the activation of MAPKs and the death of tumor cells (17). On the other hand, studies by Anand and his colleagues in 2008 showed that curcumin inhibits proliferation, invasion, angiogenesis, and metastasis in various cancers through interaction with multiple cell signaling proteins (18).
Various studies with MTT and flow cytometry confirmed that curcumin could be used in chemotherapy with and without a low-power laser. Each of them alone has an anticancer effect on colorectal cancer cells and makes the cells lead to apoptosis. Also, combining a low-power laser and curcumin at a specific dose shows a more beneficial effect.
This means that more cells have undergone apoptosis, which confirms that the effects of drugs and radiation therapy with high side effects can be reduced and replaced with a dose of natural substances without side effects, such as curcumin. Finally, it can be concluded that curcumin, if combined with other chemotherapy drugs such as low-power diode lasers, can have far greater effects in inducing cells to undergo apoptosis.