1. Background
The growth of in vitro fertilized embryos depends largely on the quality of the in vitro culture (IVC) medium (1). Embryos are primarily cultured in traditional cell culture conditions, which are unsuitable for embryo development. As a result, the use of serum in these media and the co-culture of embryos with somatic cells is common. Tissue Culture Medium-199 (TCM-199) is an example. More recently, Charles Rosenkrans medium (CR1), potassium simplex optimized medium (KSOM), and Synthetic Oviduct Fluid (SOF) have been developed for the culture of ovine, bovine, and mice embryos (2). In vitro, culture media must meet the needs of the embryos to simulate their natural movement through the fallopian tubes and uterus ("back to nature" approach). At this stage, the physiology of the embryo before and after compaction alters, thus requiring a sequential system with a different composition of the culture medium. In the "let the embryo choose" approach, it is assumed that the embryo will use whatever it needs; thus, all concentrations are concurrently utilized in a single culture medium (single-step culture medium) (3).
In other words, the proper competence of the embryo in vivo relies on the availability of nutrients in the correct amount and at the appropriate time, enabling coordination between the embryo's needs and the oviduct's environment (4). The role of serum in the in vitro embryo culture is not yet fully elucidated (5). Therefore, various supplements have been hypothesized as substitutes for FBS to provide nutrients and adhesion factors, such as human serum or plasma, cord blood serum, platelet-released factors, and platelet lysate (PL) (6). Platelets are nuclear secretory cells that respond to substances released upon vascular injury (e.g., collagen, adenosine diphosphate, and thrombin). Platelet alpha granules contain polypeptides, such as soluble adhesion molecules (e.g., vitronectin and von Willebrand factor), coagulation proteins (e.g., factor V and fibrinogen), growth factors (e.g., epidermal growth factor and platelet-derived growth factor), membrane adhesion molecules (e.g., aIIbb3 and P-selectin), and protease inhibitors (e.g., a2-antiplasmin and plasminogen activator inhibitor-1). Platelet-dense granules contain small molecules, such as magnesium and calcium, which are important for cell activation (7).
Several studies have investigated the composition of PL and have found a large number of growth factors such as insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor 2 (FGF 2), and transforming growth factor (TGF), all having a strong mitogenic effect. Also, PL contains cytokines and chemokines such as interferon (IFN) -γ, interleukin 1 L-17A, (IL)-1β, IL-2, IL-6, IL-10, IL-12p70, and tumor necrosis factor (TNF)-α (8). After activation, PL releases more than 300 substances from its intracellular granules, including a wide range of growth factors and other active molecules (9).
2. Objectives
The present study investigated using mCR2aa medium as a sequential embryo culture medium supplemented with PL to decrease FBS concentration. For this purpose, the mCR2aa culture medium was used in three steps. The serum was not used in the culture medium in the first two days (C1, 1 - 3 days of culture). From the third to fifth days of culture (C2, 3 - 5 days of culture), concentrations of 2.5% and 5% of FBS and 5% and 10% of PL were utilized. From the sixth to ninth days of culture (C3, 5 - 9 days of culture), concentrations of 2.5%, 5%, and 10% of FBS and 0%, 2.5%, 5%, and 10% of PL were employed.
3. Methods
3.1. Location
All procedures in this study were conducted in the Stem Cell and Transgenic Animals Laboratory at the Iranian Research Organization for Science and Technology (IROST), with the temperature maintained between 28°C and 30°C. All exposed surfaces were subjected to prior ultraviolet irradiation overnight.
3.2. Chemicals
Chemicals and hormones used in this study were obtained from Sigma Corporation unless otherwise noted.
3.3. Experimental Design
In the first experiment, FBS and PL were not used for the first two days of zygote culture in mCR2aa medium (C1), but for the second two days of culture, mCR2aa medium (C2) was supplemented with FBS 2.5% or 5% and PL 5% or 10%. For the remaining days of culture, mCR2aa medium (C3) was supplemented with FBS 2.5%, 5%, or 10% and PL 0%, 2.5%, 5%, or 10% (Table 1). In the second experiment, the best result of the first experiment was compared to the BO-IVC™ single-step culture medium (Table 2).
| Numbers | 3 - 5 Days of Culture | 5 - 9 Days of Culture |
|---|---|---|
| 1 | C2 (FBS 10% + PL 0%) | C3 (FBS 10% + PL 0%) |
| 2 | C2 (FBS 2.5%+ PL 5%) | C3 (FBS 2.5% + PL 0%) |
| 3 | C2 (FBS 2.5%+ PL 5%) | C3 (FBS 2.5%+ PL 2.5%) |
| 4 | C2 (FBS 2.5%+ PL 5%) | C3 (FBS 2.5%+ PL 5%) |
| 5 | C2 (FBS 2.5%+ PL 5%) | C3 (FBS 2.5%+ PL 10%) |
| 6 | C2 (FBS 2.5%+ PL 10%) | C3 (FBS 2.5% + PL 0%) |
| 7 | C2 (FBS 2.5%+ PL 10%) | C3 (FBS 2.5%+ PL 2.5%) |
| 8 | C2 (FBS 2.5%+ PL 10%) | C3 (FBS 2.5%+ PL 5%) |
| 9 | C2 (FBS 2.5%+ PL 10%) | C3 (FBS 2.5%+ PL 10%) |
| 10 | C2 (FBS 5% + PL 5%) | C3 (FBS 5% + PL 0%) |
| 11 | C2 (FBS 5% + PL 5%) | C3 (FBS 5% + PL 2.5%) |
| 12 | C2 (FBS 5% + PL 5%) | C3 (FBS 5% + PL 5%) |
| 13 | C2 (FBS 5% + PL 5%) | C3 (FBS 5% + PL 10%) |
| 14 | C2 (FBS 5% + PL 10%) | C3 (FBS 5% + PL 0%) |
| 15 | C2 (FBS 5% + PL 10%) | C3 (FBS 5% + PL 2.5%) |
| 16 | C2 (FBS 5% + PL 10%) | C3 (FBS 5% + PL 5%) |
| 17 | C2 (FBS 5% + PL 10%) | C3 (FBS 5% + PL 10%) |
test
| Numbers | 1 - 3 Days of Culture | 3 - 5 Days of Culture | 5 - 9 Days of Culture |
|---|---|---|---|
| 1 | BO-IVC™ | BO-IVC™ | BO-IVC™ |
| 2 | C1 (FBS 0% + PL 0%) | C2 (FBS 2.5%+ PL 10%) | C3 (FBS 2.5% + PL 0%) |
3.4. Oocyte Collection and In vitro Maturation (IVM)
Ovaries were collected from adult ewes at a local abattoir and transported to the laboratory in a phosphate-buffered saline (PBS) solution at approximately 37°C, supplemented with antibiotics (200 µg/mL streptomycin and 200 IU/mL penicillin) within three hours of slaughter. Cumulus-oocyte complexes (COCs) were then sliced from antral follicles. The collected contents were transferred to a heated Petri dish to isolate and classify COCs (15 oocytes per droplet) under a stereomicroscope. Following several washes, the droplets were transferred into the plate. The plates were then incubated at 38.5°C and 5% CO2 with maximum humidity for 24 hours.
3.5. In vitro Fertilization (IVF)
The presence of the first polar body indicated that the MII stage had been reached. Matured oocytes were washed three times with washing BO medium (1.942 mg/mL caffeine sodium benzoate, 10 µg/mL heparin, and 137.0 µg/mL sodium pyruvate), and 15 oocytes were transferred in individual droplets, each containing 50 µL of fertilization BO medium (washing BO medium supplemented with 10 mg/mL of fatty acid-free bovine serum albumin (BSA)). For in vitro fertilization, a warm water bath at 37°C was used to thaw the frozen semen sample for 1 minute. Then, 10 mL of BO culture medium with fatty acid-free BSA and the semen sample were placed in a sterile 12 mL test tube. This tube was centrifuged at 1400 rpm for 5 minutes at room temperature, after which the supernatant was discarded, and 5 mL of BO medium was added and centrifuged for an additional 7 minutes. Then, COCs were exposed to co-culture with a final concentration of semen of approximately 1 × 106/mL motile spermatozoa. The semen and COCs were co-incubated at 38.5°C in a 5% CO2 air atmosphere for 18 hours under 100% humidity.
3.6. In vitro Culture (IVC)
The zygotes were cultured in groups of 15 in 100 µL droplets of BO-IVC™ and mCR2aa medium, using a three-step culture system based on experimental design, without using FBS and PL during the first two days of zygote culture. From the third to fifth days, FBS 2.5% or 5% and PL 5% or 10% were used in the culture medium, which was refreshed every 48 hours. Thus, from the sixth to ninth days of culture, concentrations of 2.5%, 5%, or 10% of FBS and 0%, 2.5%, 5%, or 10% of PL were studied. Each culture droplet with 100 µL media was then covered under mineral oil. Embryos were cultured under humidified air containing 5% O2, 5% CO2, and 90% N2 at 38.5°C. The percentage of cleavage, morula, blastocysts, and hatched blastocysts were recorded on days 2, 5, 8, and 10 using a stereomicroscope.
3.7. Statistical Analysis
We utilized SPSS 16 as a statistical software program for data analysis. One-way ANOVA was used to analyze the first test, and the t-test was used to analyze the second test. The results were reported as mean ± standard error of the mean and were considered statistically significant at the P < 0.05 level.
4. Results
In this study, zygotes were cultured in the C1 culture medium for the first 48 h, as this medium was formulated to meet the requirements of the early stages of the embryo. On the second 48 h, the embryos were transferred to the C2 culture medium and then to the C3 culture medium on the remaining days, as outlined in Table 1.
4.1. Experiment 1: Investigation of mCR2aa Culture Medium with Different Concentrations of FBS and PL
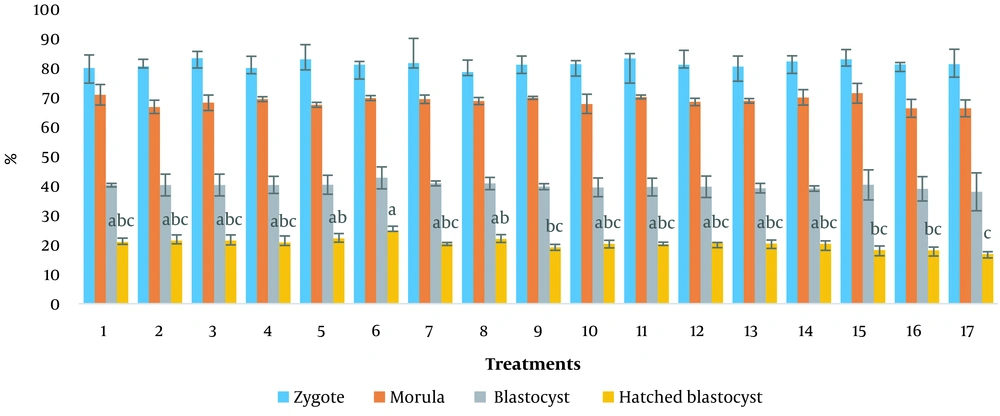
Treatment 1 containing mCR2aa medium supplemented with 10% FBS was used as a control. The developmental percentages of ovine embryos cultured in sequential media are presented in Figure 1. No significant differences were observed in the percentages of morulae produced between different treatments and the control. No statistically significant difference was observed in the blastocysts produced between the treatments and the control. However, there was a numerical difference in the number of hatched blastocysts in treatment 6 (mCR2aa + FBS 2.5%+ PL 10%, for 3 - 5 days of culture and mCR2aa + FBS 2.5%+ PL 0%, for 5 - 9 days of culture), which was 24.83 ± 1.46 (P < 0.05).
Treatments: (1) mCR2aa medium for 3 - 5 days of culture (C2)+FBS 10%, mCR2aa medium for 5 - 9 days of culture (C3)+FBS 10%; (2) C2+FBS 2.5%+ PL 5%, C3+FBS 2.5%; (3) C2+FBS 2.5%+ PL 5%, C3+FBS 2.5%+ PL 2.5%; (4) C2+FBS 2.5%+ PL 5%, C3+FBS 2.5%+ PL 5%; (5) C2+FBS 2.5%+ PL 5%, C3+FBS 2.5%+ PL 10%; (6) C2+FBS 2.5%+ PL 10%, C3+FBS 2.5%; (7) C2+FBS 2.5%+ PL 10%, C3+FBS 2.5%+ PL 2.5%; (8) C2+FBS 2.5%+ PL 10%, C3+FBS 2.5%+ PL 5%; (9) C2+FBS 2.5%+ PL 10%, C3+FBS 2.5%+ PL 10%; (10) C2+FBS 5% + PL 5%, C3+FBS 5%; (11) C2+FBS 5% + PL 5%, C3+FBS 5% + PL 2.5%; (12) C2+FBS 5% + PL 5%, C3+FBS 5% + PL 5%; (13) C2+FBS 5% + PL 5%, C3+FBS 5% + PL 10%; (14) C2+FBS 5% + PL 10%, C3+FBS 5%; (15) C2+FBS 5% + PL 10%, C3+FBS 5% + PL 2.5%; (16) C2+FBS 5% + PL 10%, C3+FBS 5% + PL 5%; (17) C2+FBS 5% + PL 10%, C3+FBS 5% + PL 10%
4.2. Experiment 2: Comparison of the Best Result from the First Experiment with BO-IVC™ As a Single-step Culture Medium
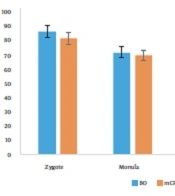
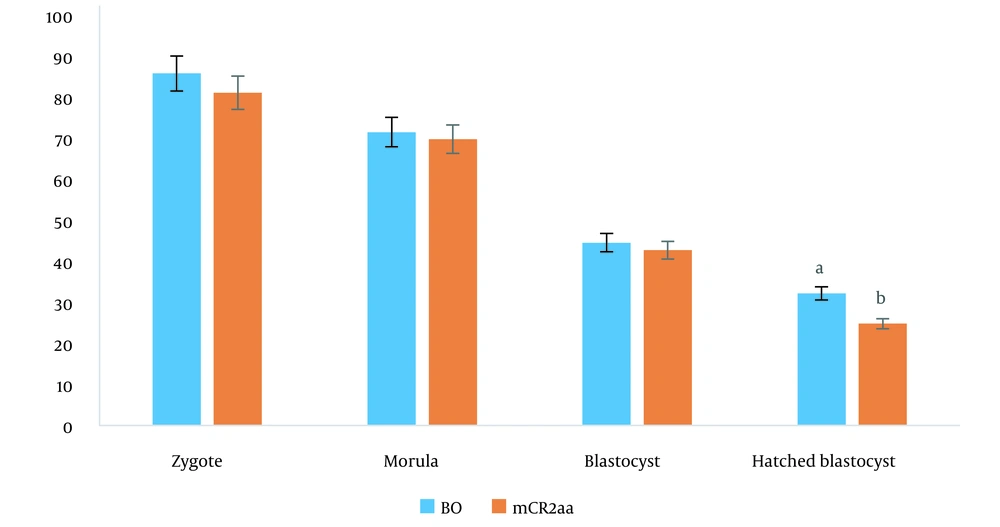
The best result of the previous experiment (mCR2aa for 3 - 5 days of culture (C2) + FBS 2.5% + PL 10%, for 5 - 9 days of culture (C3) + FBS 2.5% + PL 0%) was compared with the single-step culture medium of BO-IVC™ (Table 2). In this experiment, there was a statistically significant difference in the rate of hatched blastocysts between the single-step culture medium and the sequential culture medium, with respective percentages of 32.19% ± 1.42% and 24.83% ± 1.46% (P < 0.05) (Figure 2).
The results indicated that BO-IVC™, as a single-step medium, was associated with higher hatched blastocyst formation than the sequential three-step mCR2aa medium. Additionally, an increase in the concentration of FBS and PL in the C3 medium significantly decreased the number of hatched blastocysts (P < 0.05). Generally, when the percentage of PL was increased to 10%, the percentage of FBS needed to be reduced to 2.5% in the C2 culture medium, and the presence of PL in the C3 culture medium was not required.
5. Discussion
The growing embryo enters the uterine tube from the oviduct with different fluid compositions and environmental conditions (10). For example, the required amino acids change in the embryo. Non-essential amino acids are required up to the 8-cell stage, while all 20 amino acids are essential after morula compression (11). It has been reported that a long-term culture medium can rapidly deteriorate and, thus, be unable to support embryo development (10). Sequential media are formulated as a set of media with similar components to reduce intracellular stress during the transfer of embryos from one culture stage to the next (12). These media may involve the addition and/or subtraction of certain elements in the basal medium formulation (13). Currently, several sequential media for mammalian embryo culture are available, including Sydney IVF medium and G1/G2 medium (10), as well as Early-SOF/Late-SOF, SOFC1/SOFC2, and CDM-1/CDM-2 (13).
It has been reported that the sequential culture system is effective at the blastocyst stage, with an overall success rate comparable to that of the single-step culture medium (14). Nedambale et al. reported no significant difference in the growth rate of 8 cells or the formation of blastocysts between the KSOM-SOF sequential culture and SOF system (15). Oocytes from ovines demonstrated similar growth ability when cultured in vitro up to the blastocyst stage in sequential G1.3/G2.3 and normal SOF media (16). Embryos from bovines cultured in BSA-supplemented SOF and Sequential Quinn's Advantage Media (QAM) exhibited similar growth potential in vitro. However, the QAM medium was observed to reduce embryo quality by decreasing the total cell number of blastocysts compared to blastocysts grown in SOF media (10). Xu et al. (17) indicated that G1.2/G2.2-cultured mouse embryos had a higher quality than those cultured in KSOM or CZB. Choi et al. (18) revealed that the cleavage rates of equine zygotes cultured in either G1/G2 or DMEM, with or without BSA or 10% FBS, were similar. Swain et al. reported that using a single NCSU23 medium and sequential G1.2/G2.2 media resulted in similar fertilization percentages for pig embryos derived in vitro (19).
Moreover, sequential systems of medium for early developmental stages, followed by SOF supplemented with serum, have been reported to increase embryo growth and hatching rates in good-quality bovine embryos (20). Perin et al. reported that the percentage of blastocyst growth up to day 5 was higher in the culture of mouse zygotes in KSOM than in the G1/G2 sequential culture medium (21). The efficacy of GIII SeriesTM compared with G1.2/G2.2 has been studied in human embryos, with the rate of blastocyst formation, implantation, and pregnancy increasing compared to G1.2/G2.2 (22).
Pangestu reported no difference in mouse embryos cultured in a time-lapse incubator between single-step and sequential culture media (23). This study showed no advantage in using sequential media over single-step media. However, if laboratory conditions are carefully monitored and precautions are taken against atmospheric fluctuations, continuous single-step media culture can successfully promote growth and improve embryonic outcomes (24). However, Reed et al. randomly divided the fertilized oocytes into two culture media (single-step and sequential) and demonstrated that in the single-step culture medium, the cleavage of the embryos was faster, and the blastocysts yield was higher (24).
As to the results, using a single-step culture medium to cultivate ovine embryos is recommended. Further, human embryos cultured in single-step media (Global) were associated with significantly higher blastocyst quality and formation rates, as well as higher utilization rates, than those cultured in sequential media (ISM1/Blast Assist) (25). The advantages of a successful single-step protocol include reducing the potential for embryo loss and contamination due to management errors, reducing embryo stress due to temperature and pH fluctuations, and decreasing the cost of materials used (26).
For at least 50 years, FBS has been used as a growth supplement in cell culture media (27). As a result, there has been a major push in the field of cell culture to find alternatives to FBS (28). To this end, numerous investigators have researched potential replacements; however, a compound that is economical, simple to use, and just as effective as FBS for culturing various cell lines has yet to be identified. Platelets have been suggested as an alternative due to their high levels of growth factors (29). In this study, the percentage of serum in the culture medium was reduced, and PL was used as a substitute, resulting in a three-step culture medium. The replacement of part of the serum in the culture medium with PL showed a numerical improvement in the percentage of hatched blastocysts. To further improve the culture conditions due to the reduction of serum percentage, it is suggested that the effect of compounds such as chelating agents and antioxidants be investigated.
Non-physiological conditions in culture media and the addition of FBS have been shown to alter the expression of some genes, and there are also discrepancies in gene expression between embryos grown in laboratory conditions and those grown without serum. The expression of each gene is known to have a certain threshold, and an overabundance or dearth of expression may circumvent growth competence (30-32). Therefore, it is suggested that future research investigate the discrepancies in gene expression in embryos grown in culture media containing PL and FBS.
5.1. Conclusions
Our results showed that reducing the percentage of serum and replacing PL on days 3 - 5 of embryo culture significantly affected the formation of hatched blastocysts compared to the control with 10% serum. Thus, our findings suggest that using PL in the third step of embryo culture media is unnecessary. In light of the results obtained from the hatched blastocysts, we recommend using a single-step BO-IVC™ medium over the multi-step medium of mCR2aa to cultivate sheep embryos.