1. Background
Endometriosis is an injury caused by the proliferation of endometrial tissue outside the uterine cavity. It is one of the most common diseases in women (1) and may cause pelvic damage or impaired fertility (2). This disease results from the angiogenesis of blood vessels and the supply of oxygen and nutrients (3). Pelvic pain and infertility in women can be mostly due to endometriosis (4), which greatly impacts their living conditions. Endometriosis also affects immune system disorders, white blood cells, and the replacement of endometrial cells in the bladder and intestinal organs (5). Several treatments have been proposed to improve endometriosis, with a hormonal and metabolic basis (6). Exercise may play a role in stopping and improving this damage due to its tremendous physiological effects. In this regard, the use of vitamin D supplement may be effective in strengthening and synergizing these effects. These compounds are involved in the functioning mechanism of the muscular, nervous system and the biological cycles of energy production (7). As one of the most important vitamins, vitamin D may play a role in regulating fat metabolism and the synthesis of steroid precursor hormones. Vitamin D promotes normal cell growth (8), regulates inflammatory responses (9), increases the production of anti-inflammatory cytokines, and decreases pro-inflammatory cytokines (10, 11). This vitamin also affects the induction of apoptosis and angiogenesis (12). The importance of vitamin D supplements in endometriosis is due to the presence of vitamin D receptors and the enzymes required for its synthesis. Reports indicate that endometriosis may be due to the presence of endometrial glands and stroma in extra-uterine tissues and is estrogen-dependent. Recent research has focused on treating this disease to prevent the proliferation of stem cells and also on physical activity (13). Regular exercise can mean better endometriosis symptom management and protective effects. Exercise affects the activity of all muscles of the pelvic floor and abdominal wall by reducing estrogen. Oxidative stress is also an important factor in the physiopathology of endometriosis because reactive oxygen species are increased in the peritoneal fluid of women with endometriosis (8); moreover, regular physical activity has protective effects against diseases and inflammatory processes and increases the levels of cytokines, anti-inflammatory factors, and antioxidants, helping create and maintain the inflammatory process associated with endometriosis. Exercise may also have beneficial effects on endometriosis, with the cumulative effects of reducing menstrual flow, ovarian stimulation, and estrogen function (14). Aldehyde dehydrogenase (ALDH) is an important enzyme in the oxidation of aldehydes and is active under normal oxygen conditions. However, ALDH has been proposed to be an effective enzyme in stem cells and oxidative stress control. Therefore, changing key molecules or signaling pathways is important in oxidative damage and inflammation, and the role of the defense system will be more evident.
2. Objectives
Some evidence shows that proteins belonging to the ALDH family are involved in the pathogenesis of endometriosis as markers of normal tissue stem cells, and ALDH caused by hypoxia of cells protects against endometriosis (14). Considering the role of exercise and vitamins in health and healing diseases, this study was conducted to investigate the effects of simultaneous exercise and vitamin D3 supplement on the ALDH gene expression in endometriosis female rats.
3. Methods
3.1. Animal Model
An experimental method with a post-test design was used to conduct the research. According to the Declaration of Helsinki, all ethical issues were considered when working with animals, and the ethical charter had previously been obtained from the same faculty. The statistical population of the research consisted of three-month-old female Wistar rats (mean initial weight = 200 - 250 g). The rats were familiarized with the new environment of how to work on a treadmill and climb a ladder for seven days and kept in suitable cages with a light cycle of 12: 12 hours, an ambient temperature of 21 ± 3°C, and a humidity of 51 ± 4% with proper ventilation.
The rats were fed once every three days with a special standard scale, and according to the natural diet, 10 g per 100 g of body weight per day in each cage. At all stages of the study, the water required by the animal was freely available in a 500 mL bottle for laboratory animals.
3.2. Induction of Endometriosis
To make female rats ready for the endometriosis model, first, they were anesthetized using ketamine and xylazine. Then, the abdominal area on the right side was cleaned with betadine, and an incision was made through the skin of the intended site in the pelvic area using a scalpel blade. The abdominal muscle and peritoneum were opened, and the ovarian tissue and a part of the fallopian tube tissue were removed. The tissue was transferred and placed in a sterile container with 1 cc of polybutylene succinate (PBS). After that, each tissue was cut into pieces of 1 × 1 × 1 mm. At this stage, four pieces were considered for each rat and transplanted to the right pelvic muscle wall, abdominal peritoneum, anterior abdominal wall muscle, and fat surrounding the ovary. Afterward, the surgical area was sutured, and the rats were transferred to their respective cages. The rats were induced into the endometriosis model and divided into experimental groups. In each group, the rats were randomly divided into 6 subgroups (5 rats in each subgroup):
(1) Healthy control
(2) Endometriosis
(3) Endometriosis + Placebo
(4) Endometriosis + Vitamin D3
(5) Endometriosis + Simultaneous exercise
(6) Endometriosis + Simultaneous exercise + Vitamin D3
To eliminate the acute effects of research protocols, sampling of animal ovarian tissue is carried out 48 hours after the last exercise program. Usually, two days after the last session of the research protocols is the appropriate time to take samples from the rats’ ovarian tissues. It was also applied in this research, and sampling was carried out in completely similar conditions. For this purpose, the first female rats were anesthetized by intraperitoneal injection of ketamine (30 - 50 mg/kg) and xylazine (5 - 3 mg/kg) and then killed. The tissue samples were transferred to 10% formalin, and the samples related to gene expression were transferred to the nitrogen tank. Transplanted tissues were evaluated for histological and genetic examinations.
3.3. Vitamin D3 Supplement
Vitamin D3, a product of Zahravi Pharmaceutical Company, was purchased as a capsule from a pharmacy. The capsules were then opened with sterile scissors, poured into a graduated cylinder containing olive oil, prepared at 50 mg per kg of body weight, and given to rats daily by gavage.
3.4. Exercise Protocol
Adult female Wistar rats were kept in polycarbonate cages (5 rats per cage). Acquaintance with the treadmill for endurance exercises and climbing the ladder for resistance exercises were implemented in the animals of the designated groups for 4 days. A treadmill with automatic programming capability made by Teknik Azma (Tabriz, Iran) and a special ladder (height = 1 meter, step distance = 2 cm, and slope = 85%) made by Pi Hadi Company were used. The rats in the healthy control group had no exercise or surgery and were kept in a cage while sitting on a silent treadmill during the exercises.
The simultaneous exercise protocol was a combination of endurance and resistance exercise programs concurrently in each session. The endurance exercise program was performed at a speed of 11 m/min for 15 minutes on a rodent treadmill. Then, they did resistance exercises in the same session. The resistance exercise program consisted of going up and down the ladder (26 steps) 3 to 4 times with a weight attached to the rat's tail (15). After learning about the educational environment, the implementation of the protocol began. The exercise started with 50% of the animal's body weight. In the last session of each week, after completing the exercise program, a new one-repetition maximum (1RM) was taken to increase the exercise intensity in the following week. This protocol was implemented within eight weeks (Table 1).
| Calculating the Repetition Maximum in the First Session | Exercise Protocol |
|---|---|
| 50% body weight | 50% 1RM calculated |
| 75% body weight | 75% 1RM calculated |
| 80% body weight | 80% 1RM calculated |
| 90% body weight | 90% 1RM calculated |
| 100% body weight | 100% 1RM calculated |
| 100% body weight + 30 g 100% | 100% 1RM calculated + 30 g |
| 100% body weight + 60 g 100% | 100% 1RM calculated + 60 g |
| To reach exhaustion | To reach exhaustion |
Abbreviation: 1RM, One-repetition maximum.
3.5. Examination of ALDH2A Gene Expression
They were converted into cDNA after designing primers and RNA extraction from tissues. Then, gene expression was quantitatively converted by the polymerase chain reaction (PCR) method.
First, the RNA of whole cells was extracted using Kyazol solution according to the Synagen protocol and exposed to deoxyribonuclease I (DNase I) (Fermentas) to ensure contamination with genomic DNA. Then, the quality of the extracted RNAs was evaluated with a spectrophotometer (DPI-1, Qiagen). Oligodt primer (MWG-Biotech, Germany) and reverse transcription enzyme (Fermentas) was used to prepare single-stranded cDNA according to the relevant protocol. Each PCR reaction was performed using a PCR master mix (Applied Biosystems) and SYBER Green in an ABI StepOne Sequences Detection System (Applied Biosystems) according to the manufacturer's protocol. Forty cycles were considered for each real-time PCR (RT-PCR) cycle, and the temperature of each cycle was set at 94°C for 20 seconds, 58 - 60°C for 30 seconds, and 72°C for 30 seconds. A melting chart was used to check the accuracy of PCR reactions and was evaluated specifically for each gene and at each reaction time, along with the negative control chart, to check the presence of contamination in each reaction.
The expression ratio of the genes investigated in this study was evaluated by the cycle threshold (CT) comparative method by putting the data into the following formula:
∆∆CT = [(CTtarget - CT refence)] - (Time X) - [(CT target - CT refence)] - (Time 0)
The specific standard curve of each gene was drawn using at least 5 logarithmic concentrations in diluting order from the positive control of each gene.
The expression level of the target gene is normalized with the reference gene, and the expression of the genes of the healthy group is considered a calibrator.
(∆Ct refrence = Ct control - Ct treatment and ∆Ct target = Ct control - Ct treatment)
In the above formula, E represents efficiency, which is obtained by drawing the standard curve for the gene (Table 2).
| Primers | Sequences |
|---|---|
| r_ ALDH2A_forward | AATGGGAGAAATGGGTGAG |
| r_ ALDH2A_Reverse | TGTTGTGAGGGAAGAGTGGT |
3.6. Statistical Analysis
The descriptive statistics of mean and standard deviation were used. Moreover, the Kolmogorov-Smirnov test was used to check the normality of the data, the Lune test was used to check the homogeneity of variances, and the two-way analysis of variance (ANOVA) test was used to check the intergroup differences. In addition, Bonferroni post hoc test was used to determine significant results, and SPSS software version 23 was used to check the results (P < 0.05).
4. Results
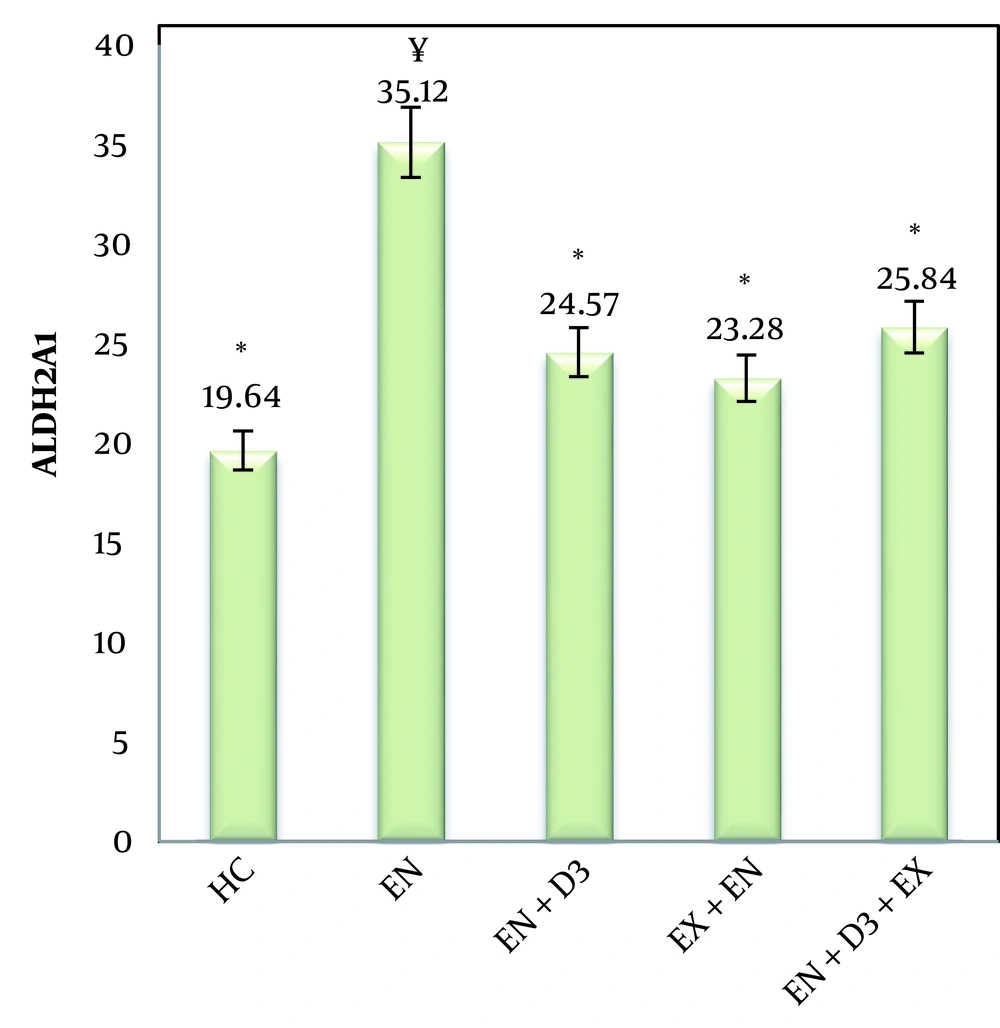
The means and standard deviations of ALDH2A values were calculated (Figure 1), and one-way ANOVA and Bonferroni post hoc tests were also used to examine ALDH2A expression in groups (Table 3). The calculated F value (F = 52.2) and its significance at the P < 0.05 level indicate a significant intergroup difference (Figure 1). According to the research findings, a significant increase in the expression of ALDH was observed in the endometriosis (disease) group. Also, compared to the healthy group, endometriosis + simultaneous exercise, endometriosis + vitamin D3, and endometriosis + simultaneous exercise + vitamin D3 did not show significant changes in ALDH activity. Therefore, it can be concluded that simultaneous exercise and vitamin D3 supplementation, individually or in combination, decreased the ALDH gene expression in female endometriosis rats.
| Variable and Groups | Significant |
|---|---|
| ALDH2A1 | |
| Control | |
| Endometriosis | 0.001 * |
| Endometriosis | |
| Endometriosis + Simultaneous exercise | 0.001 * |
| Endometriosis + Vitamin D3 | 0.001 * |
| Endometriosis+ Simultaneous exercise + Vitamin D3 | 0.001 * |
a * P < 0.05
5. Discussion
The variable investigated in this research was ALDH1A2, which decreased under the influence of vitamin D3 and endurance and resistance exercises in the supplement and exercise groups. One of the discussed mechanisms is the role of the ALDH gene in the detoxification of produced aldehydes and the oxidation of aldehydes to carboxylic acids. The results of Yildirim et al.’s studies also were similar to the findings of this research (16). Probably, the production of ALDH1A2 is inversely related to the increased expression of cytochrome P450. The decrease of this enzyme leads to decreasing the alpha-retinoic acid receptor, and finally, estradiol increases epithelial endometriosis. The secretion of this enzyme is active under normal oxygen conditions and catalyzes the conversion of nitrate compounds into nitric oxide, so it is natural that with the increase of these aldehydes, endometriosis increases; consequently, the changes in ALDH2 activity may be the basis of endometriosis disease pathology. The increase in ALDH2 activity reduces the allogenic input to the central nervous system by sensory afferents of the lesion to reduce pain. Therefore, the reduction of this enzyme in the supplement and exercise groups compared to the endometriosis group is a sign of lesion reduction (17). According to the available reports, vitamin D3 reduces the growth and implantation of endometrial tissue, and the abnormal concentration of vitamin D3 causes the incomplete removal of endometrial cells passing through the peritoneum and ovarian reflux, which is another effective mechanism in the results of this research (17). In Chung et al.’s research, high levels of vitamin D3 and calcium have been confirmed in endometriosis (18). A serum concentration of more than 70 ng/mL increases the growth of endometriosis, which is contrary to the results of this research. High levels of calciferol increase the risk of endometriosis, but in cysts that have already developed, it is a strong inhibitor of re-angiogenesis (19, 20). In addition, hypovitaminosis D3 is also a potential risk factor for endometriosis. Exercise may help improve endometriosis symptoms (reduced menstrual cycles and bleeding between periods, reduced pain and constipation, increased energy, and improved sleep). The effect of physical activity on endometriosis is through the effect of endorphins as a natural painkiller. It also boosts mood and controls anxiety and depression, likely due to the constant pain and changing hormones, and increased estrogen levels in endometriosis. Lack of sleep can increase inflammation and anxiety and worsen endometriosis. It can be said that endometriosis is related to pelvic floor dysfunction. Sports activities, particularly resistance exercises, improve the strength and condition of body muscles. Increasing mobility through endurance activities relaxes muscles and reduces hip pain. There are also reports of the management of gastrointestinal symptoms of constipation, bloating, and irritable bowel syndrome with exercise. Some research has linked fatigue to endometriosis. Fatigue is often associated with sleep problems, depression, and pain. However, staying active helps some women regain their energy. Body movement increases blood flow, which equals more energy. These results are consistent with the findings of Tibana et al.'s study (15). The results of Ghasemian et al.’s study investigating the effects of aerobic swimming and vitamin B6 on the GATA index (21) are inconsistent with the findings of the present study by reporting that regular aerobic exercise, as well as co-administration of vitamin B6, may affect GATA2 gene expression (20). Chung et al. have shown that a diet rich in vitamins and antioxidants is effective in the metastasis and growth of endometriosis (18). Of course, the antioxidant and anti-inflammatory roles of vitamin D3 and its contribution to strengthening the immune system can be effective in reducing endometriosis (20). In this regard, it can be said that endometriosis feeds the estrogen hormone and leads to inflammation, bloating, and pelvic pain, but exercise helps reduce estrogen. Reducing the risk of endometriosis and focusing on exercise are useful approaches, causing the release of myokines from the muscles. These results are consistent with the findings of Augostinis et al.’s study (22). In addition, exercise increases the production of leukocytes, cortisol, and adrenaline, all of which have metabolic, neurological, and inflammatory effects (23). There is a relationship between regular and vigorous exercise and its effect on health (24). Progressive overload in sports has stronger effects, and a reduction in stress levels has been reported (25). Moreno et al. also achieved these results in their research. Pelvic floor muscle tension in women with endometriosis pain is higher than the control group without endometriosis (26). Which are possibly effective mechanisms for reducing endometriosis in this research? There is still no definitive treatment for endometriosis, and the main focus is on pain control and hormonal suppression. The role of physical activity and exercise has been suggested as part of the therapeutic approach. Inflammation caused by endometriosis sensitizes pelvic organs (13, 27); although inflammatory factors were not investigated in this research, physical activity is acceptable to reduce inflammation, prevent disease progression, and improve pain (28). The type, intensity, and duration of exercise, medical history, and the type and amount of vitamins and supplements consumed seem to be effective on the severity of endometriosis and the differences in findings. Therefore, exercise and supplements can contribute to reducing and stopping endometriosis.
At present, the cause and initial history of endometriosis are not clear, treatment methods are few, and no definitive treatment has been recognized for this disabling disease. However, it is known that angiogenesis considerably contributes to endometriosis pathogenesis (13, 27). Angiogenesis inhibitors have been indeed suggested as a new treatment method for this disease, as they have been demonstrated to suppress endometriotic lesion growth in an animal model (28). It is worth mentioning that using cDNA microarrays for the investigation of endometriosis encounters some restrictions. Endometriosis tissue is heterologous, i.e., it consists of a combination of endometrium, myometrium, serosa, connective tissue, and immune cells. Hence, no one can guarantee that the profiles of the current study’s gene expression stem from a tissue that contributes to the pathophysiology of this disease).
5.1. Conclusions
The role of the ALDH gene in the detoxification of aldehydes produced by endometriosis and the conversion of nitrate compounds into nitric oxide are significant. Hypovitaminosis D3 is also a potential risk factor for endometriosis. Exercise may help improve symptoms of endometriosis through endorphin effects as a natural painkiller, reducing digestive disorders, increasing blood flow, leukocyte production, and health effects.