1. Context
Muscle hypertrophy is probably one of the most important goals of resistance training (RT). Most people who do resistance exercises seek to increase muscle mass and then muscle performance, especially muscle strength (1). Resistance training is the most common training program for developing strength and stimulating muscle hypertrophy. This type of training improves muscle strength by increasing the cross-sectional area of the muscle caused by hypertrophy (2). Note that although creating hypertrophy is one of the main goals of bodybuilders and competitive athletes, muscle hypertrophy or maintaining muscle mass has therapeutic applications for non-athletes, too, because the reduction of muscle mass is closely associated with the occurrence of some underlying diseases (3). That is why RT, with the approach of muscle hypertrophy, is highly recommended to everyone, including athletes and non-athletes. To obtain the desired outcome in muscle adaptations caused by RT, the correct design of RT components, including intensity, duration, number of repetitions and sets, and duration of rest periods between sets, is of great importance (4). This is because these components can increase protein synthesis in skeletal muscles and cause hypertrophy by activating signaling pathways (5). Spiering et al. (2008) believe that muscle hypertrophy caused by RT results from a series of interrelated events, including muscle activation, signaling events caused by mechanical deformation of muscle fibers, hormones, and immune/inflammatory responses; another reason is protein synthesis that occurs because of increased transcription and translation; the last reason is fiber hypertrophy, which occurs consecutively (6). Lim et al. (2022) pointed out that skeletal muscle hypertrophy caused by RT depends on external variables such as the characteristics of the RT program, the designated diet, some supplements, and internal variables such as mechanical transport, ribosomes, gene expression, and satellite cell activity (7).
The available evidence indicates that the use of natural substances, nutritional diets, supplements, and vitamins can alter genes, proteins, and the overall physiological homeostasis of the body (8-10). There is substantiated evidence that vitamin D plays an important role in muscle hypertrophy. Bass et al. (2020) showed that the overexpression of the vitamin D receptor in skeletal muscles increased protein synthesis in myofibrils by raising anabolic signaling, especially the mammalian target of rapamycin (mTOR), which can be observed in the form of muscle hypertrophy (11). Closely consistent with this study, Bass et al. (2021) reported that the knockdown of the vitamin D receptor in vitro and in vivo increases skeletal muscle atrophy. Knockdown of the vitamin D receptor of the muscle caused atrophy by decreasing the total protein content, which reduced the myofiber level. Finally, vitamin D receptor (VDR) knockdown disrupted myogenesis (cell cycle, differentiation, and myotube formation) in vitro. These results show the importance of VDRs and signaling pathways in maintaining skeletal muscle protein (12). Nonetheless, evidence suggests that skeletal muscle is a direct target for vitamin D, and it is widely reported that vitamin D plays a determining role in regenerating human skeletal muscles. It is also a contributing factor in the maintenance of serum 25(OH)D, which might be necessary for increasing the repair processes and potentially facilitates the hypertrophy process (13). Since both RT and vitamin D affect muscle hypertrophy, this review aimed to summarize the existing knowledge about the simultaneous effect of RT and vitamin D on muscle hypertrophy in more depth and detail.
2. Evidence Acquisition
2.1. Date and Mode of Searching the Articles
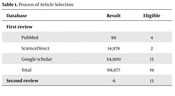
Three databases (PubMed, ScienceDirect, and Google Scholar) were searched until September 9, 2022 (Table 1). Only original articles in English were reviewed. The main distinguishing factor in the analysis of this review was that the selected articles dealt with both exercise and vitamin D to investigate skeletal muscle hypertrophy in more depth and detail.
| Database | Result | Eligible |
|---|---|---|
| First review | ||
| PubMed | 99 | 4 |
| ScienceDirect | 14,978 | 2 |
| Google scholar | 54,900 | 13 |
| Total | 69,977 | 19 |
| Second review | -6 | 13 |
Process of Article Selection
2.2. Searched Keywords
Exercise AND vitamin D AND hypertrophy OR training AND vitamin D AND hypertrophy OR resistance AND vitamin D AND hypertrophy
3. Results
The process of selecting the most relevant articles involved meticulous cross-checking and elimination of cases that did not meet the criteria. The search results from different databases provided valuable insights. For instance, PubMed yielded 99 results, and after a thorough screening, 4 articles (13-16) were found to be eligible. ScienceDirect produced a substantial number of results, totaling 14,978. After a meticulous evaluation, 2 articles (17, 18) were deemed suitable. Google Scholar, with its extensive database, generated a significant number of results, amounting to 54,900. Following a rigorous assessment, 13 articles (19-31) were selected for inclusion.
The selected articles underwent an extensive review process to ensure their reliability and accuracy. Each article was read and scrutinized multiple times to thoroughly understand its content and implications. However, during this rigorous evaluation, it became evident that certain articles needed to be excluded for specific reasons. For example, 1 article focused primarily on the human heart rather than skeletal muscle (15), which did not align with the objectives of the present study. Additionally, several articles did not directly address the topic of hypertrophy (18, 19, 29). Furthermore, 1 article specifically investigated cardiac fibrosis (20), and another article solely discussed muscle morphology (21), topics that were not directly relevant to the research question.
After careful consideration of these factors and the elimination of articles that did not meet the criteria, the remaining studies were categorized into two distinct sections: Cross-sectional area and muscle performance. This division allowed for a comprehensive analysis of the selected studies and facilitated a deeper understanding of the impact of RT interventions and vitamin D on muscle hypertrophy.
3.1. Cross-Sectional Area
Agergaard et al. (2015) showed that 12 weeks of RT and vitamin D intake increased the cross-sectional area of the quadriceps muscle in young and elderly men (14). Holm et al. (2008) reported an increase in muscle mass in women after 24 weeks of RT and supplementation (protein, carbohydrate, calcium, and vitamin D) (16). Owens et al. (2015) found that in human skeletal muscle biopsied in vitro, 10 nmol of 1α, 25(OH) 2D3 improved the migration dynamics of muscle cells and improved myotube fusion/differentiation at the biochemical, morphological, and molecular levels. Their result showed an increase in myotube hypertrophy in 7 and 10 days after the injury (13). Kim et al. (2020) showed in p62-deficient obese mice that 10 weeks of RT with 1000 IU of vitamin D3/kg/d had a significant effect on hindlimb muscle wet weights and myofiber cross-sectional area (17). Visser et al. (2003) showed that 25-hydroxyvitamin D is associated with sarcopenia in elderly men and women (32). Daly (2010) investigated the independent and combined effects of exercise and vitamin D on muscle morphology, function, and falls in the elderly. They found that exercise and vitamin D combination had a synergistic effect on the function and morphology of muscle (21). According to Daly et al. (2014), whey protein drinks with vitamin D supplementation can augment the effects of RT on increasing muscle volume and size in elderly people with type 2 diabetes (22). After 12 weeks of RT and 8000 IU daily of vitamin D, Savolainen et al. (2021) observed a significant increase in total and regional lean mass, while similar results were observed in the placebo group (23). In this regard, Molmen et al. (2021) showed that RT increased muscle mass in men and women, and vitamin D did not affect the impact of RT on muscle mass (24). According to Chen et al. (2022), in young men, 6 weeks of RT, along with receiving whey protein and vitamin D, causes a significant elevation in muscle mass compared to RT alone (25). Hofmann et al. (2016) found that in elderly women, RT, along with a food supplement containing protein, carbohydrates, and vitamin D, caused muscle growth through the insulin-like growth factor-1 (IGF-1) signaling pathway (26). In a similar study, Kukuljan et al. (2009) reported that 18 months of RT in healthy men aged 50 to 79 years increases the cross-sectional area of the mid femur muscle, and the consumption of milk enriched with vitamin D and calcium enhances the effect of RT (27). Based on Amorim et al. (2018), in people with spinal cord injury, both creatine and vitamin D, together with exercise, increase the arm muscle area (28). Sisi et al. (2021) showed that simultaneous interventions (vitamin D, calcium, and resistance exercise) cause morphological changes in the soleus muscle, including an increase in the number and diameter of muscle fibers and a decrease in the thickness of the endomysium in postmenopausal rats (30). According to Motavari et al. (2022), in male futsal players with vitamin D deficiency, supplementation with vitamin D3, along with RT, increases muscle volume (31).
Based on the studies mentioned, RT combined with vitamin D supplementation exerts positive effects on muscle mass and morphology. The findings indicate that RT and vitamin D intake can increase the cross-sectional area of muscles, improve muscle cell migration dynamics, enhance myotube fusion/differentiation, and promote muscle growth. These effects are observed in different populations, including young and elderly individuals, women, individuals with type 2 diabetes, and those with spinal cord injury. Additionally, the combination of vitamin D with other nutrients such as protein, carbohydrates, calcium, and creatine may further enhance the effects of RT on muscle volume and size. It is also suggested that maintaining adequate levels of 25-hydroxyvitamin D is important for preventing sarcopenia in the elderly. Overall, the evidence supports the potential benefits of combining RT with vitamin D supplementation for optimizing muscle mass and morphology.
3.2. Muscle Performance
Owens et al. (2015) showed that 48 hours and 7 days after a traumatic extensor knee extension activity, vitamin D3 supplementation improved maximum torque recovery (13). According to Agergaard et al. (2015), after 12 weeks of RT, isometric strength increased in young and elderly men, but no significant difference was observed between vitamin D3 and placebo groups. The strength increase was significantly higher in the elderly group than in the young group (14). Holm et al. (2008) showed that 24 weeks of protein, carbohydrate, and vitamin D supplementation in postmenopausal women significantly raised concentric and isokinetic muscle strength (16). In a study on p62-deficient obese mice, Kim et al. (2020) demonstrated that muscle function, including grip strength and sensorimotor function, did not significantly change during 10 weeks of exercise and vitamin D intervention (17). Visser et al. (2003) reported decreased grip strength in elderly men and women with low serum 25-hydroxyvitamin D levels compared to those with higher 25-hydroxyvitamin D levels (32). Savolainen et al. (2021) reported the lack of effect of vitamin D along with RT on young men suffering from vitamin D deficiency (23). According to Molmen et al. (2021), after 13 weeks of RT and vitamin D consumption, vitamin D did not affect the increase in strength caused by RT (24). Chen et al. (2022) reported an elevation in muscle strength in the leg press movement after 6 weeks of RT and receiving a combination of protein and vitamin D (25). Hofmann et al. (2016) reported a promotion in muscle strength (handgrip) after 6 months of RT with a food supplement containing protein, carbohydrates, and vitamin D in elderly women (26). According to Kukuljan et al. (2009), vitamin D-enriched milk did not affect muscle strength along with RT in elderly men (27). Amorim et al. (2018) observed a significant relationship between serum vitamin D levels and one repetition maximum of Pec Deck (28).
Based on the reviewed studies, the effects of vitamin D supplementation on muscle strength appear to be mixed. Owens et al. (2015) (13) demonstrated that vitamin D3 supplementation improved maximum torque recovery after a traumatic activity. Agergaard et al. (2015) (14) showed an increase in isometric strength after 12 weeks of RT in both young and elderly men, but no significant difference was observed between the vitamin D3 and placebo groups. However, the elderly group showed a significantly higher increase in strength compared to the young group. According to Holm et al. (2008), protein, carbohydrate, and vitamin D supplementation in postmenopausal women led to significant increases in concentric and isokinetic muscle strength (16). On the other hand, Kim et al. (2020) found no significant changes in muscle function, including grip strength and sensorimotor function, with exercise and vitamin D intervention in p62-deficient obese mice (17). Visser et al. (2003) observed decreased grip strength in elderly individuals with low serum 25-hydroxyvitamin D levels compared to those with higher levels (32). Savolainen et al. (2021) (23)and Molmen et al. (2021) (24) reported no significant impact of vitamin D supplementation on muscle strength in young men and people undergoing RT, respectively. However, Chen et al. (2022) (25) demonstrated increased muscle strength in the leg press movement with a combination of protein and vitamin D supplementation during RT. Finally, Hofmann et al. (2016) (26) reported an increase in muscle strength (handgrip) in the elderly.
4. Discussion
Skeletal muscle is a dynamic tissue, and its size and cross-sectional area are affected by various factors. One of the most important external factors playing a role in skeletal muscle hypertrophy is mechanical load, the most common type of which is RT. Evidence shows that RT leads to hypertrophy by increasing muscle protein synthesis (33). The increase in protein synthesis caused by RT is the result of a series of cascade reactions activated by growth factors and anabolic hormones, especially androgens and IGF-1. In other words, RT activates the process of intracellular signaling for protein synthesis by increasing the release of anabolic hormones (34). Protein synthesis in skeletal muscles is regulated by a complex biological network of intracellular signaling mechanisms. The IGF-1/PI3K/Akt signaling pathway is one of the most effective molecular signaling systems for muscle growth and hypertrophy. This pathway plays a key role in the hypertrophic process because it carefully regulates the molecular basis of protein degradation and synthesis (35). In this pathway, Akt (protein kinase B) plays a central role in the signaling pathway of muscle hypertrophy and atrophy (36). Various stimuli, such as growth factors, cytokines, and hormones, activate AKT. The activation of AKT plays a critical role in muscle protein synthesis, followed by muscle hypertrophy so that, in skeletal muscle, the expression of the active isoform of Akt1 leads to myotube hypertrophy in vitro and in vivo (37). The activation of AKT activates the mTOR (mammalian target of rapamycin) pathway and the GSK3β (glycogen synthase kinase-3 beta) pathway, which play an important role in skeletal muscle hypertrophy (38). The activation of mTOR by AKT causes the phosphorylation and activation of p70S6K, as well as the phosphorylation and release of the inhibitory effect of PHAS-1/4E-BP1, while the activation of GSK-3β by AKT inhibits the inhibitory effect of eIF2B on protein synthesis (39). Baar and Esser (1999) found that RT activates p70S6K; moreover, the close relationship between p70S6K and the increase in muscle mass indicates that mTOR-induced p70 (S6k) phosphorylation may be a good marker for muscle hypertrophy and could be involved in muscle growth. The skeleton caused by the biomechanics of resistance exercises may also play a role (40). It has also been reported that the induction of rapamycin as an mTOR inhibitor inhibits muscle hypertrophy caused by RT (41). Besides, RT inhibits eIF2B immediately and 3 hours after exercise by activating GSK-3β AKT and stimulates the process of protein synthesis in skeletal muscles (42). Overall, RT leads to hypertrophy by increasing muscle protein synthesis, which is regulated by a complex network of intracellular signaling mechanisms mentioned above. These complex interplay of signaling pathways and protein synthesis mechanisms contribute to the hypertrophic impacts of RT on skeletal muscle.
Another mechanism by which exercise can affect skeletal muscle hypertrophy is increasing insulin sensitivity and improving the insulin signaling pathway in skeletal muscle. It has been reported that RT in prediabetic obese people increases the expression of phosphorylated Akt2, mTOR, and muscle hypertrophy (43). Consitt et al. (2013) also reported a rise in Akt2 protein expression in young and old people after RT (44). Holton et al. (2004) showed that 6 weeks of RT with one leg increased the expression of insulin receptor protein and Akt in the active leg compared to the inactive leg, indicating an adaptation in the insulin signaling pathway in skeletal muscles following RT (45). In general, RT improves the insulin signaling pathway in skeletal muscles, and in this way, it prevents muscle protein destruction and muscle atrophy. Therefore, resistance exercises can exert their effects on hypertrophy or muscle mass maintenance.
Skeletal muscles are the direct target of vitamin D. Vitamin D has been implicated in human skeletal muscle regeneration. Evidence suggests that maintaining serum 25(OH) D may be beneficial for enhancing repair processes and potentially facilitating hypertrophy (13). The presence of vitamin D receptors in skeletal muscles shows that this vitamin is essential for muscle function and metabolism (46). Low levels of vitamin D (VDR) are associated with skeletal muscle fiber atrophy, muscle pain, weakness, and increased risk of sarcopenia (47). Vitamin D affects muscle strength, muscle function, and muscle metabolism due to changes in protein synthesis, myogenesis, mitochondrial activity, muscle regeneration, and glucose metabolism in muscles (13). Vitamin D, like RT, can stimulate IGF-1 and thereby exert its anabolic effect on skeletal muscle tissues (48). Tanaka et al. demonstrated that myoblasts rely on signals transmitted through VDR in order to undergo differentiation into myocytes. They also highlighted the significance of VDR expression in skeletal muscles for the preservation of muscle volume in older individuals (49). In addition, the serum 25(OH) D3 level and VDR expression in muscle cells decrease with age, which is consistent with the decline in muscle mass and the incidence of sarcopenia caused by aging (50). Significant muscle atrophy, decreased muscle strength, reduced muscle fiber size, lower bone density, and disturbance in the regulation of myogenic regulatory factors were observed in mice in which VDR has been knocked out and in mice that received a diet with a low vitamin D content, compared to the control group (51, 52). It has been reported that vitamin D increases the phosphorylation of Akt and GSK3β and thus improves insulin signaling by regulating the insulin receptor, which itself can activate the protein synthesis process in skeletal muscle (53). Since vitamin D has an antioxidant effect, evidence shows that in conditions of vitamin D deficiency, the enzyme antioxidant defense is reduced and increases proteolysis in skeletal muscle tissue (54). The results of numerous studies show that vitamin D improves protein synthesis and hypertrophy in C2C12 muscle cells (55). Furthermore, vitamin D influences the production and secretion of insulin, affects the survival of β-cells of the pancreas, and increases the secretion of insulin from β-cells of the pancreas (56, 57). Vitamin D not only affects the production and secretion of insulin from pancreatic beta cells but also influences the response of peripheral tissues to insulin or, in other words, the insulin signaling pathway in skeletal muscles (53). It has been reported that vitamin D raises insulin sensitivity by binding 1,25(OH) 2D3 to VDR, inducing insulin-receptor substrate expression in target tissues and activating PPARδ (58). In confirmation of the effect of vitamin D on the insulin signaling pathway in peripheral tissues, evidence suggests that in conditions of vitamin D deficiency, insulin resistance is observed due to the down-expression of the insulin receptor (59). Besides, supplementation with 1,25(OH)2D3 improves glucose metabolism by regulating the SIRT1/IRS1/GLUT4 signaling cascade and glucose uptake in C2C12 myotubes (60). Another mechanism for the effect of vitamin D on improving insulin function and reducing insulin resistance in skeletal muscle is its impact on the regulation of intracellular calcium ions, which causes the transfer of GLUT4 from the depth of muscle cells to the cell membrane and glucose absorption; as a result, the rate of glucose uptake by skeletal muscle increases (60). Since insulin signaling through the activation of the PI3k/AKT/mTOR signaling pathway causes the maintenance and development of muscle hypertrophy, evidence shows that the lack of insulin signaling in skeletal muscles is associated with a decline in muscle mass and muscle function, which can justify the reduction of intramuscular protein synthesis (61). Therefore, the reduction of vitamin D can harm the process of muscle hypertrophy through the disruption of the insulin signaling pathway. Both RT and vitamin D can activate the signaling pathways in muscle cells that increase protein synthesis, develop hypertrophy, and promote muscle function, particularly in areas such as muscle strength; thus, the simultaneous use of these two interventions can be a suitable solution for strengthening the impact of each on muscle hypertrophy, preventing muscle atrophy, and enhancing sports performance in athletes. Since both of these interventions can inhibit oxidative stress, inflammation, and apoptosis, in addition to the stimulating signaling pathway of muscle hypertrophy, they can be used as an efficient method for people suffering from muscle-wasting diseases such as diabetes and cancer and in the elderly. They can also be used to mitigate the complications of the aforementioned diseases. In conclusion, vitamin D plays a crucial role in skeletal muscle function, regeneration, and metabolism. Low levels of vitamin D are associated with muscle atrophy, weakness, and a higher risk of sarcopenia. Vitamin D affects muscle strength, function, and metabolism by influencing protein synthesis, myogenesis, mitochondrial activity, muscle regeneration, and glucose metabolism. It can stimulate IGF-1 and exert an anabolic effect on skeletal muscle tissue. The presence of vitamin D receptors in skeletal muscle highlights its importance for muscle volume maintenance.
Generally, Studies have shown that vitamin D deficiency leads to muscle atrophy, decreased muscle strength, and disturbances in myogenic regulatory factors. Vitamin D improves insulin signaling, increases insulin sensitivity, and affects glucose metabolism. It also regulates intracellular calcium ions, which enhance glucose uptake by skeletal muscle. The activation of the insulin signaling pathway is crucial for muscle hypertrophy and protein synthesis. Both RT and vitamin D can activate signaling pathways that increase protein synthesis and promote muscle hypertrophy. Combining these interventions may enhance their effects on muscle hypertrophy, prevent muscle atrophy, and enhance sports performance. Additionally, RT and vitamin D can reduce oxidative stress, inflammation, and apoptosis, which makes them potential therapeutic strategies for muscle-wasting conditions such as diabetes, cancer, and aging-related muscle loss.
4.1. Conclusions
In summary, examining the studies exploring the simultaneous impact of RT and vitamin D on hypertrophy and skeletal muscle function revealed that the studies are very limited (about 13 studies). The effect of RT and vitamin D on signaling pathways of protein synthesis and skeletal muscle hypertrophy was investigated alone, and the simultaneous impact of these two interventions was studied in a few studies. Since the proteins involved in the signaling pathway of muscle hypertrophy were examined in only 3 animal studies, more field/clinical trials in the human model should investigate the influence of these two interventions on the degree of hypertrophy and muscle function. Besides, both in human studies using muscle biopsy and in animal studies at the cellular level, the expression of genes and proteins involved in signaling pathways affecting protein synthesis and muscle hypertrophy should be studied. In this way, the simultaneous effect of these two interventions can be explored in depth and detail.