1. Background
The pancreas is a complex organ that contains both exocrine and endocrine functions and secretes the hormone insulin (1). Chronic organ dysfunction is effectively treated with organ transplantation. Organ transplantation is a procedure for repairing and replacing damaged tissues or organs. However, it is frequently hampered by a scarcity of donor tissues/organs and immunological concerns caused by infectious infections (2). Diabetes mellitus is the world's most common, dangerous, and epidemic metabolic illness (3, 4). Diabetes affects approximately 347 million people worldwide and poses a severe threat to their health. The incidence of diabetes is rapidly increasing due to population expansion, aging, obesity, and sedentary lifestyles (4). This disease is also one of the diseases of the pancreas tissue that is related to the hormone insulin. If needed, one of the solutions for treating this disease is a pancreas transplant, which, like other body organ transplants, has many problems (4).
Transplanting the pancreas is a well-established operation that outperforms islet transplantation in terms of long-term metabolic performance. The reason is that islet cell transplantation may result in portal vein hypertension or embolization. However, indications for pancreas transplantation are limited and significant, and sometimes life-threatening problems such as post-transplant pancreatitis, infections, and thrombosis continue to cause approximately 10% graft loss (3, 5). With the development of novel tissue engineering techniques based on stem cells and scaffolds (eg, decellularization and recellularization), the above-mentioned issues could be overcome and open a new window in organ or tissue replacement (3, 6).
Engineering human tissues is currently a multidisciplinary and active topic of study (7). Transplantation medicine is an area of applied medical research that is only getting started (2). Regenerative medicine is a large branch in which certain types of cells, primarily stem cells, are employed to repair damaged tissues or organs. The ultimate objective of regenerative medicine is to repair and establish normal organ functioning (8). Although the criteria that bioengineered cells must meet to be regarded as viable therapy have yet to be developed, any possible therapy must be both safe and functionally superior to current therapies (1). Decellularization is a process used in tissue engineering to separate the extracellular matrix (ECM) from the cells in a tissue. This process can lead to the creation of natural scaffolds that are used in the construction of artificial organs and tissue repair (9). The extracellular matrix is a heterogeneous connective network in native tissues and organs that functions in vivo to provide the structural support, mechanical stability, and biochemical signals necessary for tissue morphogenesis and homeostasis. It comprises fibrous glycoproteins, proteoglycans, and small molecules. Fully recreating the ECM features is a huge challenge (7). Decellularization makes it possible to get cell-free, natural ECMs.
The preservation of the 3D structure, which provides support, tensile strength, and attachment sites for cell surface receptors, and the availability of bioactive components that regulate angiogenesis, cell migration, cell proliferation, and cell orientation in wound healing are the 2 main characteristics that explain why decellularized ECM scaffolds can support tissue regeneration (7). Mesenchymal stem cells (MSCs) may be obtained from various sources and rapidly grown in vitro. Researchers have recently discovered that umbilical cord Wharton's jelly is an ideal source for isolating MSCs since it includes wholly defined MSCs with strong proliferation rates. These cells are hypoimmunogenic and release growth factors (8).
Natural polymers, in contrast to synthetic scaffolds, offer superior biocompatibility since acidic byproducts are not formed during decomposition, resulting in no unwanted inflammatory reaction. An alternate approach to treating pancreatic diseases may be possible through the construction of a bioengineered pancreas using the proper combination of cells, biomaterial scaffolds, and physiologically active chemicals (10). In this regard, in tissue engineering and regenerative medicine, to help with problems caused by pancreas transplantation, MSCs and their growth in decellularized scaffolds of pancreatic tissue are used to regenerate the desired tissue. Therefore, the best alternative to pancreas transplantation for clinical use is the combination of pancreatic decellularization scaffold and stem cells (9, 11).
2. Objectives
The purpose of this research was to prepare a scaffold from natural pancreatic tissue by decellularization method, evaluate the suitability of the rat pancreas scaffold for MSC culture, and check the survival of these cells on the scaffolds.
3. Methods
3.1. Pancreas Decellularization
This study was approved under the ethical approval code of IR.UMA.REC.1400.023.
To prepare the rat's pancreatic cell scaffold, 25 adult male Wister rats weighing 230 - 270 g were purchased and kept in the animal house of Mohaghegh Ardabili University, Faculty of Basic Sciences. According to Mohaghegh Ardabili University's Ethics Committee's endorsement, the rat was sacrificed in a special chamber with an atmosphere containing carbon dioxide. The pancreatic tissue was surgically and carefully dissected to separate it from the surrounding organs, such as the stomach, colon, spleen, and mesenteric tissue. In this study, a healthy pancreas was extracted from 3 animals to be used as native tissue. After cleaning the sample from extra tissues, it was placed in a phosphate-buffered saline (PBS) solution and transferred to the temperature of 4°C for decellularization.
The preparation steps of the pancreas decellularization scaffold were as follows:
1- Immersion: The pancreatic tissue was immersed in distilled water for a duration of 5 h.
2- Ionic detergent: The tissue samples were then placed in a 0.5% sodium dodecyl sulfate (SDS) solution, an ionic detergent, for a period of 12 h.
3- Distilled water wash: After the tissues became translucent, they were washed with distilled water for 15 min.
4- Triton X-100 solution: The tissue samples were transferred to a 1% Triton X-100 solution for a duration of 24 h.
5- Distilled water wash: The target tissue was then washed and re-washed with distilled water for 15 min.
After performing the steps, the decellularized samples were kept in a PBS buffer solution with pH = 7.2 at 4°C.
All decellularization steps were performed at room temperature.
3.2. Histological Analyses
The effectiveness of the decellularization method was evaluated using the hematoxylin and eosin (H&E) staining technique in terms of the removal of cells and the presence of nuclear remnants in histology. Briefly, the decellularized pancreas was fixed with 10% formalin. Then, dehydration was done by grading, increasing the concentration of ethanol, and then xylene. Paraffin-embedded tissues were cut at 4-μm thickness, and the tissue sections were deparaffinized with xylene and graded with decreasing ethanol concentration. Slices were stained with H&E and Masson's trichrome to examine the morphology of the intact and acellular pancreas tissue. In the slides stained with H&E, the extracellular fibers are pink under the light microscope, and in Masson's trichrome staining, the nucleus is usually black, and the collagen layers are blue. Masson's trichrome tissue staining is used to see collagen fibers specifically. DAPI staining was used to confirm the removal of cells and examine the residual DNA in the sample of decellularized scaffolds. The DAPI dye binds to 2 pairs of adenine and thymine strands on 2 DNA strands and in a DNA minor groove. Fluorescent blue is visible under UV light.
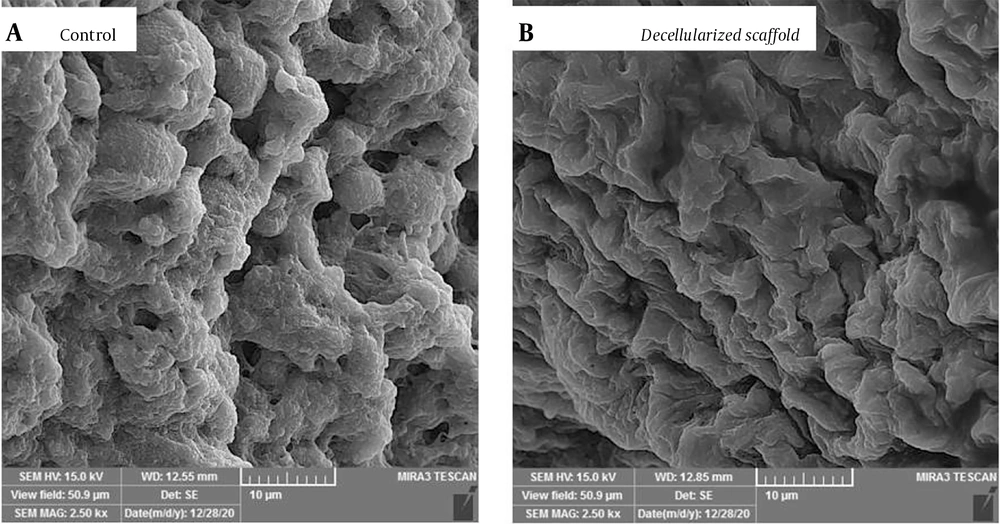
3.3. Decellularized Pancreas Microstructure Analysis by Scanning Electron Microscopy
To examine the exact changes in the level of the decellularized scaffold structure with the intact pancreas tissue (control) with the help of a scanning electron microscope (SEM), briefly, first, the samples were fixed by 2.5% glutaraldehyde for 2 h. Then, dehydration was done with an increasing percentage of ethanol alcohol. After drying, the samples were covered with gold-palladium coating and prepared for imaging and examination using an SEM.
3.4. DNA Quantification
DNA extraction was used to evaluate the remaining DNA in the decellularized and intact pancreas. The amount of DNA in native and decellularized tissues was compared by a DNA quantification kit to measure DNA content according to the manufacturer's instructions. DNA isolation is based on cell lysis, followed by selective DNA precipitation. DNA was extracted using the Synaclone extraction kit, and for evaluation, the concentration spectrometry of a DNA nanodroplet at 260 nm was used. A NanoDrop spectrophotometer was also used to determine the purity of nucleic acids by comparing the absorbance at 260 and 280 nm. Briefly, 100 μL of protease buffer was added to 30 mg of healthy and decellularized pancreatic tissue. Then, 5 μL of protease was added and incubated at 55°C for 1.5 h (until lysis), and then DNA extraction was performed according to the Synaclone kit protocol. Finally, to calculate the DNA concentration relative to the weight of the tissue, the obtained concentration was divided by the weight of the tissue used (ng/mg).
3.5. Sulphated Glycosaminoglycan Content Characterization
All decellularized samples were examined using tissue-specific staining, and the glycosaminoglycan (GAG) content was calculated to validate the preservation of ECM integrity. Glycosaminoglycans play an essential role in the construction of ECM, where they contribute to tissue strength and, in cooperation with collagen, maintain the structure of ECM. To measure GAGs, first, the samples were analyzed by enzymatic digestion using the Kiasyst kit to release the GAG content, and the prepared solutions were transferred to a 96-well plate. Then, in the presence of reactants, a color was produced, read by a microplate spectrophotometer, and absorbed at a wavelength of 510 to 560 nm. The GAG content was determined using a microplate spectrophotometer based on a standard curve created using the standard GAG kit. Reading of the absorbed wavelength was repeated 3 times for every sample.
3.6. Seeding Adipose Mesenchymal Stem Cells to Assess Cytocompatibility and Cell Distribution
The succinate dehydrogenase enzyme reduces and breaks down tetrazolium crystals in the MTT test, resulting in the formation of blue, insoluble crystals. An MTT experiment was carried out to assess cell viability in the decellularized pancreatic. Briefly, 96-well microplates containing adipose MSCs were incubated overnight at 37°C in a humid environment containing 5% CO2. Adipose MSCs were injected into the decellularized pancreas scaffold using an insulin syringe. Then, the plate was placed in a CO2 incubator at 37°C for 24 h. Then, 200 μL of 0.5 mg MTT was added to each well. After 4 h in the dark phase, 10 μL of DMSO was added to each well, and finally, the amount of light absorption at the wavelength of 630 was read by a microplate reader. Absorbance values are related to the number of living cells. Wells without biological scaffolds were considered as controls.
3.7. Amount of Total Protein by the BCA Method
The BCA protein measurement is a method to measure the amount of protein in tissue. The principle of the method is that proteins can reduce Cu2+ to Cu+ in an alkaline environment, which is known as the Biureh reaction, and a purple color is produced in the presence of bicinchoninic acid. This BCA method is more accurate compared to the Bradford method and the reason is that all chains of peptide bonds participate in the formation of color. Another advantage over the Bradford method is that reagents, such as NP-40, Triton X-100, or SDS, do not interfere.
3.8. Statistical Analysis
SPSS version 16.0 was used to conduct the statistical analysis.
Results are reported as SD; each test was run 3 - 6 times.
4. Results
4.1. Macroscopic Observations During Decellularization
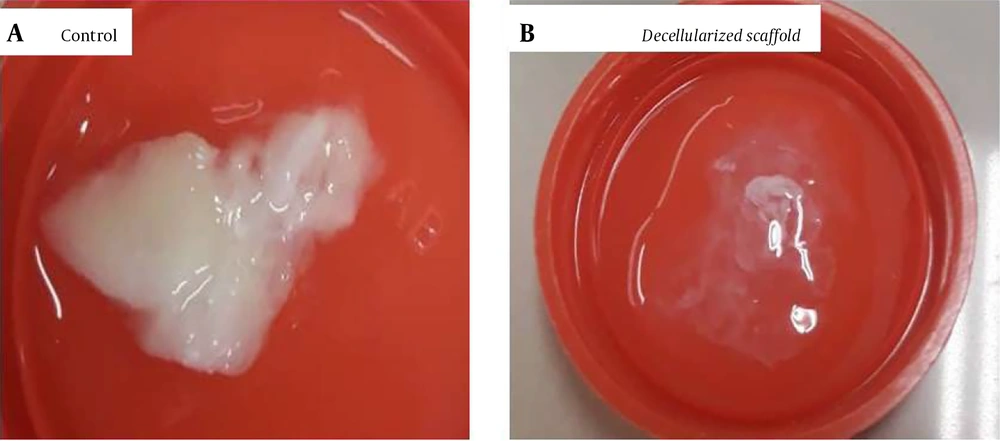
At first, the technique of physical decellularization of freezing and thawing cycles was performed, which did not bring any specific results. Next, with the completion of chemical decellularization by anion-based detergent (SDS 0.05% and Triton X-100), the morphology of the pancreas tissue was thoroughly evaluated. The decellularized pancreas showed a significant color change (Figure 1B), and a transparent appearance was observed in the evaluation.
In general, macroscopically, a gradual color change was observed during the decellularization process. The produced scaffolds were completely decellularized, but the microgrid remained intact. At the end of the decellularization process, the structure of the organ was still preserved, but the cellular components were removed. The decellularization process continued until the entire pancreas was completely clear. Finally, as shown in Figure 1, the anionic (0.05%) SDS-based detergent concentration caused tissue degradation and disrupted tissue integrity, producing a transparent appearance. After removing the main cellular components of the native pancreas (Figure 1A), the decellularized matrix of the pancreas (Figure 1B) has a different and more straightforward appearance than the control pancreas, indicating proper decellularization.
4.2. Extracellular Matrix Scaffold Composition
4.2.1. Histological Evaluation of Decellularized Matrices
All decellularized samples were examined using tissue-specific staining (Figure 2).
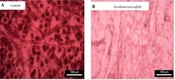
The hematoxylin and eosin (H&E) staining image of the A, control pancreas and ; B, decellularized pancreas at 10x magnification. The results of H&E staining and checking the general morphology of fresh rat pancreas and acellular pancreas showed that decellularization was complete, and cell removal was visible in the decellularized pancreatic tissue sample.
The results of H&E staining revealed that decellularization was complete, and the decellularized pancreas was entirely free of cells and nuclear materials. Therefore, after the completion of decellularization, cell nuclei were not present in the decellularized pancreas, and no cellular material was stained in the decellularized matrix (Figure 2B).
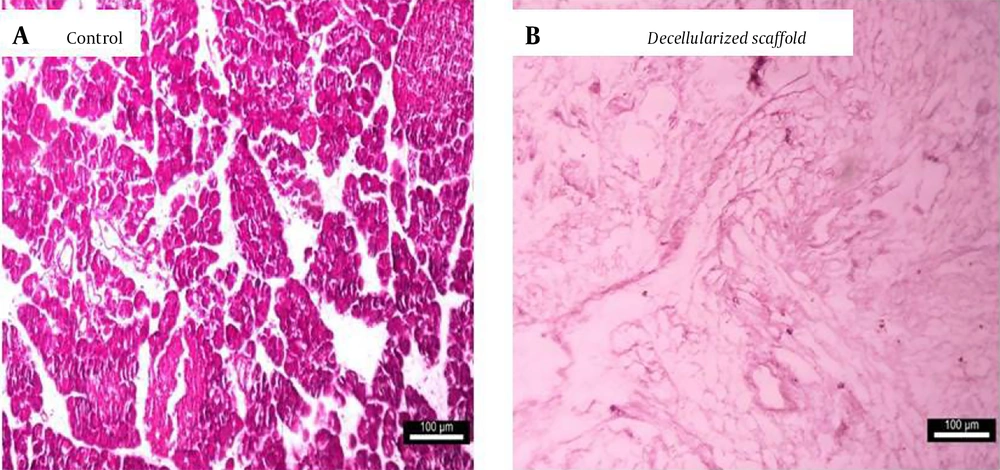
Additionally, Mason trichrome staining was performed to visualize collagen fibers as abundant components of ECM. They showed that structural components of the pancreas (collagen and elastin fibers) were conserved in the prepared decellularized pancreas (Figure 3).
The image of Mason trichrome staining and comparison of extracellular matrix preservation of the A, intact pancreas and; B, decellularized pancreas of rats at 40x magnification. The results of Mason trichrome staining showed that the structural components of the pancreas (collagen and elastin fibers) were conserved.
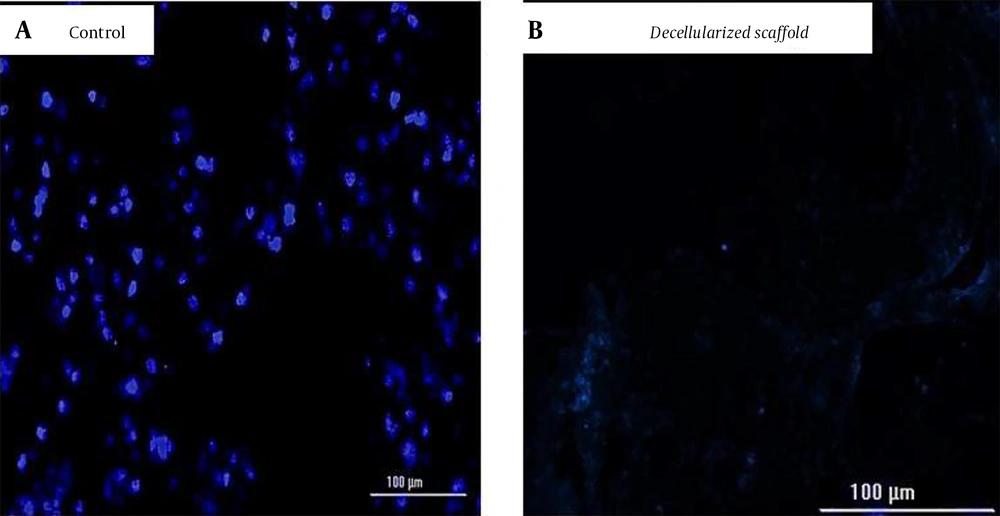
In the results of Masson trichrome staining, preservation of the ECM, primarily collagen, can be seen in both decellularized (Figure 3B) and intact (control; Figure 3A) pancreas. Collagen was maintained following decellularization, indicating that our decellularization technique effectively eliminated all cellular components while still preserving the makeup of the ECM. Additionally, the decellularized pancreatic scaffold's ECM structure resembled that of a natural pancreas. Microscopically, no relevant differences were observed between the experimental groups (Figure 3), and the decellularized ECM appeared identical to the controls. The DAPI staining of specimens after the decellularization process showed the complete removal of cells. No residual DNA and cell nuclei could be observed in the decellularized group compared to the intact pancreas (Figure 4).
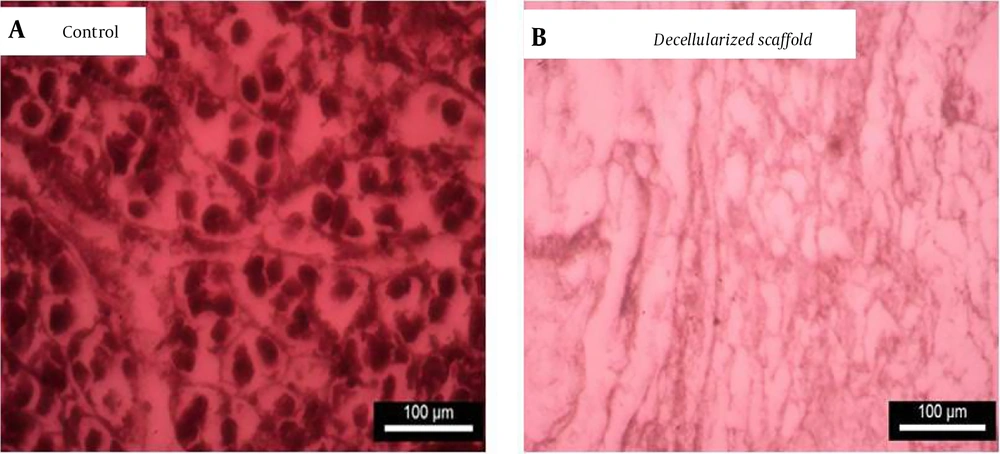
The image of the DAPI staining of the pancreas of rats. The DAPI-stained cross sections of the rat pancreas were used to compare the cell nuclei of the A, freshly dissected pancreas and ; B, decellularized pancreas segment. The results showed that the cell nucleuses disappeared in the decellularized pancreas.
In comparison to the native pancreas (Figure 4A), DAPI staining showed that no cellular material was present in the decellularized scaffold (Figure 4B). This result implies that our decellularization technique retains the composition of the ECM while entirely removing all of the cellular components. In general, DAPI staining showed that decellularization could remove all cellular elements.
4.3. Glycosaminoglycans and Collagens
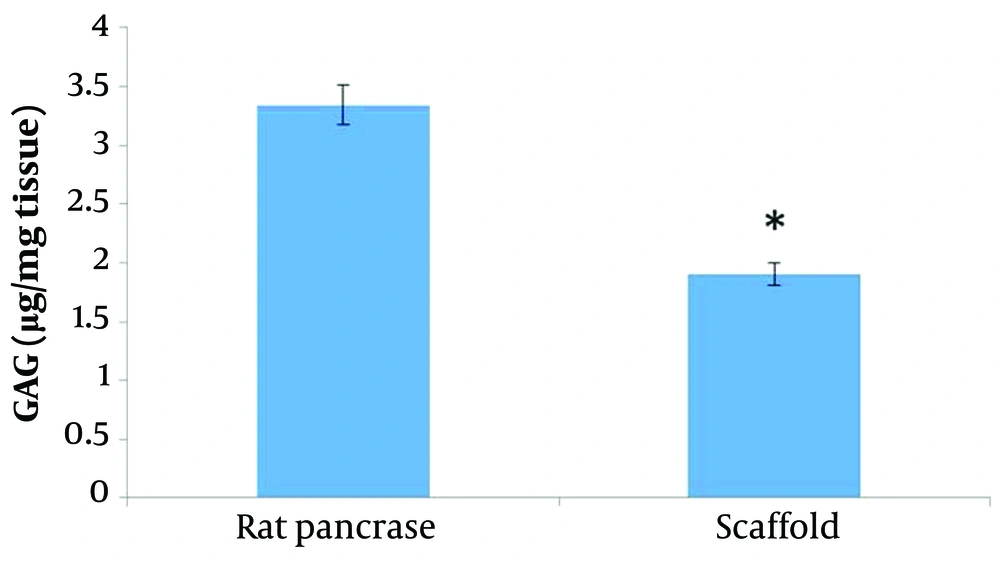
The amounts of GAG were calculated to confirm the preservation of ECM integrity. A biochemical study was conducted to demonstrate the preservation of the residual GAG content in the matrix. We aimed to quantitatively analyze and validate the retention of the primary ECM components. The GAG content was quantified using the Kiazist GAG assay kit by the manufacturer's instructions, and the results demonstrate no significant difference between the amount of GAG in the scaffold of the pancreas and the control pancreas, indicating that GAG is preserved in the ECM (Figure 5). In fact, bioscaffolds maintained more fabulous GAG material.
4.4. DNA Quantification
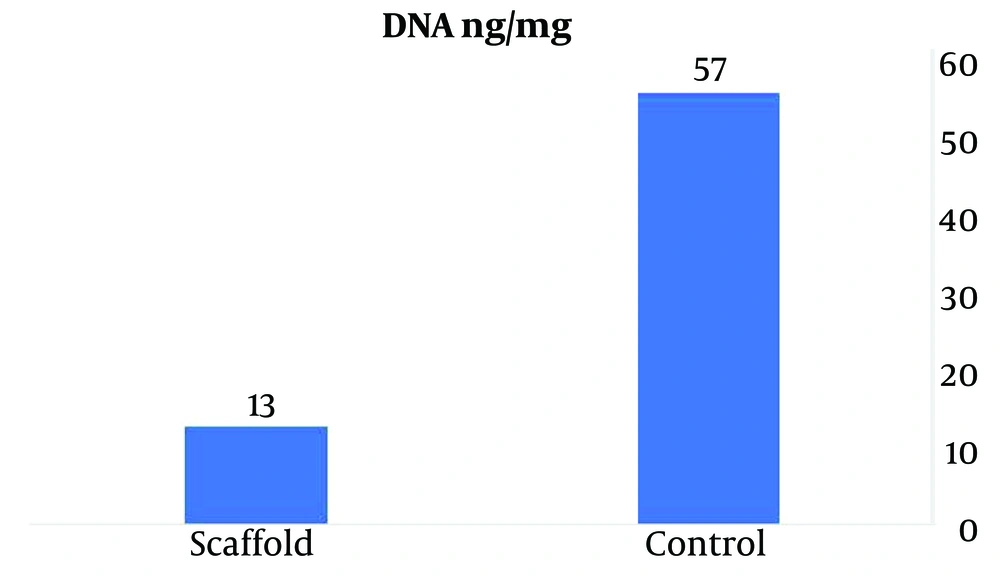
DNA quantification was carried out to further evaluate the effectiveness of decellularization. In a decellularized pancreas, the total DNA content declined significantly compared with the controls. Quantifying of DNA content was done before and after decellularization treatment using the SinaClon kit to verify the efficacy of the process and confirmed effective decellularization in the bioscaffold. Compared to a normal pancreas, double-stranded DNA decreased in the decellularized pancreas, indicating successful cell elimination. The results of comparing the amount of DNA in healthy pancreatic samples with the decellularized pancreatic detected that the amount of DNA significantly was reduced from 57 ng/mg in the native pancreas to 13 ng/mg in the decellularized pancreatic, indicating the removal of cells from the pancreatic tissue. These findings strongly suggest that the decellularization process of the pancreas was successful in effectively eliminating its cellular components (Figure 6).
4.5. Total Protein Content
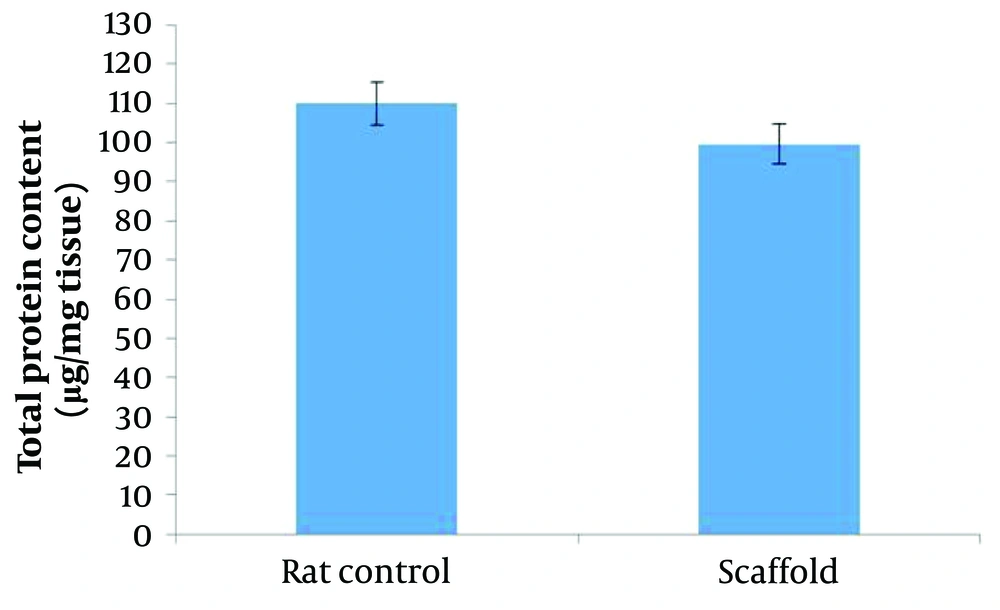
The results related to the examination of the amount of protein showed the similarity of the amount of total protein in the decellularized scaffold with the intact pancreatic tissue, and no significant decrease was observed in the amount of protein in the decellularized pancreas compared to the control pancreas. In general, in this experiment, as much as the amount of total protein in the ECM of the decellularized tissue was closer to the ECM of the control tissue, the decellularization was done better (Figure 7).
4.6. Scanning Electron Microscopy
To confirm the decellularization process, the prepared scaffold was examined with an electron microscope (Figure 8).
The results related to the ultrastructural evaluation of the decellularized sample showed the complete removal of cells from the scaffold and the preservation of the ECM structure. The decellularized pancreas scaffolds' tissue integrity and microarchitecture were retained; as seen in Figure 8B, a biological porous scaffold with a homogenous structure was made from the voids left behind by cell detachment. This scaffold is suitable for cell seeding.
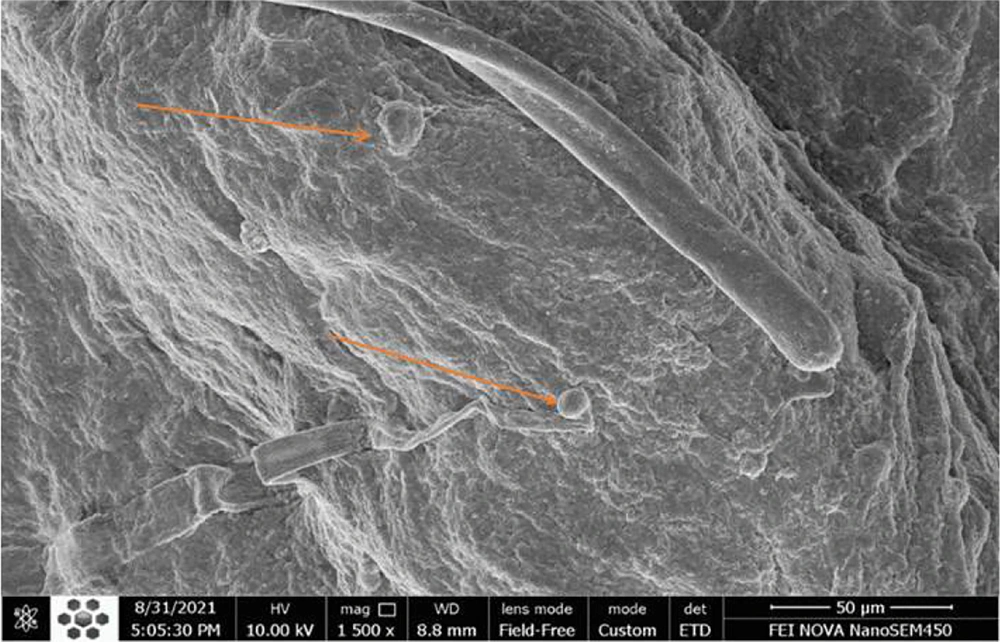
4.7. Cell Adhesion to the Decellularized Scaffold Surface by a Scanning Electron Microscopy and Cell Viability
Adipose MSCs' adherence and maintenance to the decellularized scaffold of rat pancreas by electron microscope were evaluated and studied in vitro. By examining the results of the prepared image, the adhesion of the cells on the scaffold can be recognized (Figure 9).
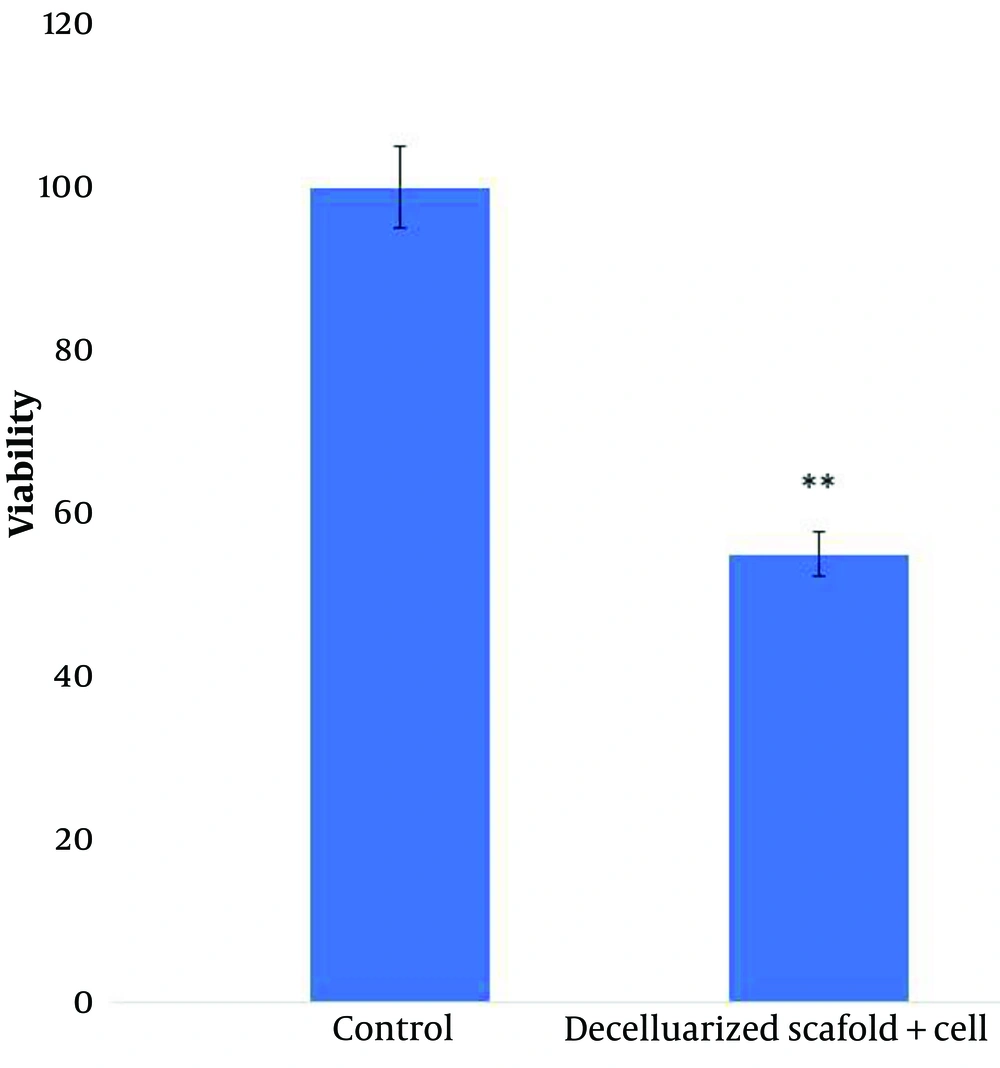
The MTT assay showed that adipose MSCs cultured in the decellularized pancreas displayed viability comparable to the positive control, indicating that the bioscaffold was not cytotoxic and that cells may proliferate there. This means that the scaffold of a decellularized pancreas showed signs of cell survival and proliferation after 8 days of culture, but the evaluation of the results of cell viability on different scaffolds showed a significant decrease in cell viability on decellularized scaffolds compared to the control group (P < 0.01). Therefore, the growth and proliferation of fat MSCs also depend on environmental factors (Figure 10).
The viability and proliferation of MSCs were investigated after culture. The results showed that MSCs survived in the decellularized scaffold from pancreatic tissue. The experiments were performed in triplicate.
5. Discussion
To completely destroy all cellular components, some decellularization methods are used, such as melting and freezing cycles, which are known as physical methods and mechanical stimulation. However, this method does not guarantee the preservation of the ECM microstructure, which is essential for tissue engineering (9, 12). Extracellular matrix proteins support angiogenesis in addition to cell transport, proliferation, and differentiation (9). Extracellular matrix components, as well as 3D- and microstructure and physical characteristics, were retained in our approach for complete organ decellularization. Data from Masson trichrome staining showed that the collagen composition of the decellularized pancreatic scaffold was identical to that of the natural pancreas.
In pancreatic, diabetes mellitus causes a decrease in function and/or absolute numbers of insulin-producing cells. Islet transplantation is being studied as a potential solution, and developments in tissue engineering approaches can help increase pancreatic islet survival and performance. Transplanted pancreatic tissue develops anoikis, hypoxia, and an immunological response driven by inflammation, resulting in early graft damage and failure (12). The need for transplantation and repair of pancreatic tissue is one of the biggest human concerns due to the many problems in organ transplantation, and scientists are always looking for ways with fewer complications for organ transplantation (13). Decellularized organs can now be used as scaffolds for cell treatments because of recent advances in tissue engineering. This is because it can preserve natural ECM, and the vascular, decellularized pancreas might be a suitable substrate (14).
Tissue engineering and regenerative medicine have increased the use of biological materials by providing new strategies to solve organ transplant problems and suggesting innovative ways to develop new treatments. Providing suitable conditions for better repair, regeneration, and proliferation of cells is done by pancreatic tissue engineering scaffolds (13). More challenging than 2-dimensional culture is whole organ bioengineering because it requires a complicated and cell-supporting framework to distribute nutrients to all organ parts. The 3D structure is critical for subsequent cell survival and performance (13, 14). Clinical trials have been conducted with bioengineered tissues and organs, such as synthetic bladders, tracheas, nasal cartilage, and tubular tissue (15, 16).
For the first time, several organs (such as the heart, lung, and liver) were decellularized after coronary arteries were decellularized (17-19). Successful decellularization includes eliminating all cells and maintaining ECM components and a vasculature that resembles that of the original tissue. Rat, pig, and human pancreases have all been subjected to various decellularization methods (18, 19). Therefore, one of the biggest concerns among tissue engineering and regenerative medicine scientists is to perform the decellularization technique with minor damage to the ECM while preserving it and removing the maximum number of cells. The decellularization technique, which has recently been used in tissue engineering by removing all cellular material from an organ, can provide us with a 3D biocompatible scaffold; thus, stem cells can be grown in it (20).
Physical techniques (such as thawing and freezing cycles) and chemical techniques (such as the use of detergents) are used in tissue decellularization (21). Examining and evaluating the results of decellularization showed that the cellular components of the control pancreas were removed acceptably; the general framework of the tissue and biomechanical properties, which are essential in scaffolds, were preserved, which is a sign of successful decellularization. Goh et al., who had performed the same method of pancreatic decellularization, achieved similar results in H&E staining of the scaffolds obtained from this research (22, 23).
The scanning electron microscopy showed that a 3-dimensional network of collagen fibers remained from decellularization, collagen being the most abundant pancreatic ECM protein. Data from Masson trichrome staining showed that the collagen composition of the decellularized pancreatic scaffold was identical to that of the natural pancreas (24). While alkaline and acid therapies can eliminate biological components and nucleic acids like RNA and DNA, they can degrade ECM components like GAGs (25). Nonionic detergents (such as Triton X-100) affect lipid-lipid and lipid-protein interactions, although they are intended to retain protein cell particles while destroying the basement membrane (26). The ionic detergent SDS can remove nuclear remnants and cytoplasmic proteins altogether. While effectively dissolves cell membranes, the native tissue structure with minimal damage to the ECM is mostly preserved by this detergent (27).
Studies have shown that damage to any of the components of the ECM, such as collagen, reduces the mechanical strength of tissue engineering scaffolds. In this case, the shelf life of stem cells in such scaffolds is surprisingly reduced. The use of trypsin for decellularization has been shown to cause harm to ECM components (28).
In Guo et al.'s study, decellularization of the whole pancreas was performed using perfusion with Triton X-100 and NH4OH solution. In our study, Triton X-100 was used to effectively remove nuclear and cytoplasmic fragments from SDS, as well as to affect lipid-lipid and lipid-protein interactions (29).
In recent studies, it was shown that a mouse pancreatic bioscaffold based on SDS/Triton perfused through the anterior hepatic portal vein might regenerate the natural pancreas for pancreatic tissue engineering (30). The goal of this study was to use a technique to generate a decellularized pancreatic matrix for pancreas tissue engineering using a mix of Triton and SDS.
In the study by Sevastianov et al., Triton X-100 and SDS were used to decellularize pancreatic tissue, and the results showed that the pancreatic tissue was well decellularized and the basic proteins of the ECM were well preserved (31). Therefore, the technique allowed total cell removal while maintaining the ECM structure (sGAG and collagen content) and vasculature.
The results showed that the ECM of the decellularized pancreatic scaffold provided a suitable microenvironment for cell adhesion. The adipose MSCs survived in these scaffolds and have high durability. Based on our findings, it was observed that the pancreas became translucent, and a higher percentage of the remaining DNA was eliminated.
5.1. Conclusions
This research showed that the decellularized pancreas scaffolds, whose decellularization was done correctly and efficiently according to the results obtained, considering the integrity of the cells in the scaffold, can be a suitable platform for the growth of MSCs.
This study can significantly contribute to the development of engineered pancreas tissue transplantation and provide an innovative approach for tissue engineering experiments and regenerative medicine. Therefore, it can be further investigated as an ideal scaffold in future research, especially in in vivo conditions.