1. Background
Menopause is defined as the absence of a menstrual cycle for 12 consecutive months, marking the end of the child-bearing years, typically around 40 - 50 years of age (1, 2). Menopause results from hormonal changes that lead to menstrual cessation and accounts for almost a third of a woman's life (3). Estrogen (Est) deficiency, which occurs after menopause, induces abnormalities such as metabolic diseases, decreased bone mineral density (BMD), and premature cell aging (4). Estrogen plays an important role in maintaining bone strength; however, the onset of menopause is marked by declining Est levels, leading to increased bone loss (5). This physiological phenomenon and the mechanisms underlying Est decline are better understood experimentally in animal models via ovariectomy (OVX), where the ovaries are removed (6). The removal of the ovaries can affect the transcription of proteins responsible for maintaining BMD.
In both mice and humans, the degradation of bones is governed by the activity of peroxisome proliferator-activated receptor gamma (PPAR-γ) (7). PPARβ/δ plays a crucial role in regulating bone turnover and the communication between osteoblasts and osteoclasts. Unlike PPARγ activation, PPARβ/δ reduces the process of osteoclastogenesis mediated by osteoblasts (7). Osteoblast differentiation and bone formation require osterix (OSX), an essential transcription factor (8).
Alkaline phosphatase (ALP) is a homodimeric protein that can add phosphate groups to other molecules. Alkaline phosphatase exists in the serum in almost equal amounts as two isoforms: Tissue-specific ALP and liver-specific ALP (BALP) (9). Biologically, BALP adheres to the osteoblast cell membrane and releases a small amount into the serum. BALP serum concentration rises only during bone remodeling (9, 10). BALP stimulates tissue mineralization mainly by inactivating pyrophosphate and osteopontin, both of which are mineralization inhibitors. High and low ALP concentrations post-menopause indicate greater and reduced BMD, respectively (9).
Estrogen replacement therapy can provide relief from menopausal symptoms, yet it could potentially heighten the likelihood of developing breast cancer, heart disease, or a blood clot in the lungs (11). As a result, there has been a growing focus on physical activity and the use of supplements as alternatives to Est replacement therapy. Resistance training (RT) to improve muscular strength and muscle mass is a recommended non-pharmacological approach to mitigate the negative physiological changes associated with menopause (12). Additionally, researchers have demonstrated that supplementation with vitamin D (Vit D) and calcium (Ca++) can have beneficial effects on menopausal symptoms (13, 14). Vitamin D can modulate cancer and inhibit apoptosis in menopausal conditions (15). In human and rat osteoblasts, Vit D stimulates differentiation and mineralization (16). In metabolic syndrome, Vit D has been shown to increase PPAR-γ expression and enhance the benefits of physical activity (17). However, there is limited data available regarding the impact of vitamin D on the OSX and ALP genes.
Chitosan (Chit) is a dietary fiber derived from the cuticles of crustaceans, including shrimp, crab, lobster, and mushrooms (18). Previous research has demonstrated that Chit alleviates menopausal symptoms such as weight gain, visceral fat content, and tail skin temperature in Est-deficient rats (19). This may be because acetylated Chit oligosaccharides act as antagonists against cell death (20). Mathews et al. (21) found that Chit enhanced the mineralization process during osteoblast differentiation of mesenchymal stem cells derived from human bone marrow by upregulating several genes such as osteopontin, osteocalcin, osteonectin, collagen type 1 alpha 1, and integrin-binding sialoprotein (21); however, this did not include PPARγ and OSX genes.
Information regarding the influence of Vit D, Ca++, and Chit intake on PPAR-γ, OSX, ALP, osteoblast, and osteoclast factors post-menopause is currently lacking. Further, literature on comparing Est replacement therapy with RT and Vit D, Ca++, and Chit interventions is also insufficient.
2. Objectives
Therefore, the purpose of this study was to investigate the impact of RT, Vit D, Ca++, and Chit on post-menopausal changes in bone turnover markers, and their possible synergistic effects in comparison to Est replacement therapy in OVX rats.
3. Methods
3.1. Animals, Groups, and Ethical Considerations
Forty-two female Wistar rats were randomly selected and divided equally into seven groups of six. One group represented the healthy control (He-Co) to serve as a reference for menopause effects. The other six groups were OVX and placed in the following categories: (1) OVX-Vit D + Ca++ + Chit + RT; (2) OVX-saline (Sal) + Est + RT; (3) OVX-Sal + RT; (4) OVX-Vit D + Ca++ + Chit; (5) OVX-Sal + Est; and (6) OVX-Co (Figure 1).
The research followed all the rules for ethical animal care set by the Ministry of Health and Medical Education. The study design adhered to the guidelines set by the Ministry of Health for Animal Studies and received approval from the Central Tehran Branch, Islamic Azad University, Iran ethical committee.
3.2. Ovariectomy Procedures
Thirty-six female rats, aged eight weeks, were anesthetized to establish the model. They were given ketamine (30 - 50 mg/kg, Sigma-Aldrich, USA) and xylazine (3 - 5 mg/kg, Sigma-Aldrich, USA). The hair was shaved from the lumbar region, and the ovaries were surgically removed. The wounds were sutured, and the rats were given one month to heal and start experiencing menopause (22). The rats were then divided into the previously mentioned intervention groups.
3.3. Training Protocol
The selected groups (1), OVX-Vit D + Ca++ +Chit + RT; (2) OVX-saline (Sal) + Est + RT; (3) OVX-Sal + RT) underwent a familiarization period with the ladder RT protocol (three nonconsecutive days, six times per session, without load). Upon completion, they performed the RT protocol intervention (three nonconsecutive days per week for six weeks) based on the principle of overloading, beginning with 75% body weight and progressing to 90% body weight. The rats performed the RT protocol, which consisted of six required ascents with one minute of rest between ascents (23).
3.4. Supplementation Procedures and Estrogen Induction
Supplement creation, supplementation, and Est injection were performed according to Dehghan-Manshadi et al. (24). The supplementation was provided orally at 100 mg/kg body weight immediately following each training session for six weeks. Estrogen (Sigma-Aldrich, USA) was dissolved in corn oil at a concentration of 5 μg/kg body weight. This solution, totaling 100 μL, was injected subcutaneously into each rat three times per week over a period of six weeks.
3.5. Determination of Gene Expression
Femur samples were analyzed using real-time polymerase chain reaction (RT-PCR) to investigate gene expression. Special kits were used for each stage of the analysis, including RNA extraction, cDNA synthesis, and RT-PCR (25). Total RNA was extracted from the samples and subsequently converted into cDNA. The cDNA was amplified using RT-PCR, and the cycle threshold (CT) values of the genes were recorded. These CT values were then converted into relative gene expression data. The primer sequences are presented in Table 1.
| Gene | Forward | Reverse |
|---|---|---|
| PPAR-γ | CTCAGGCAGATTGTCACA | CAGCGACTGGGACTTTTC |
| ALP | CCCACAGCTTCAGTTCCCCCTCA | CACCTACAATACCACTGCCACAC |
| OSX | GGGGGCATTTGGTTAGGTGGT | GGTGGGGTGTTGGATAGGGAG |
| GAPDH | AAGTGATGGAGATGAAGGAGT | CAGGCGTGAATGATGAAGAGT |
Abbreviations: GAPDH, glyceraldehyde-3-Phosphate dehydrogenase; OSX, osterix; ALP, alkaline phosphatase; PPAR-γ, peroxisome proliferator-activated receptor gamma.
3.6. Histological Assays
Histological assays using hematoxylin and eosin (H&E) staining were conducted to investigate tissue alterations and structural changes among different animal groups. Once the treatments were completed, all rats were euthanized under anesthesia. The targeted tissues were then removed and fixed in 4% paraformaldehyde. Tissue samples were prepared and dehydrated using a series of alcohol concentrations, cleared with xylene, and embedded in paraffin. Subsequently, a microtome was used to create 5-µm thick sections, which were placed onto albumin-adhesive microscope slides. The tissue sections then underwent deparaffinization, clearing, and hydration procedures. Staining of the sections involved hematoxylin for seven seconds, followed by eosin for three minutes. These stained tissue samples were observed under a light microscope (Nikon, Tokyo, Japan) and analyzed using ImageJ software.
3.7. Statistical Analysis
The data were reported as mean ± standard deviation. An independent t-test was used to determine differences between the He-Co and OVX-Co groups to assess the effect of OVX. Two-way ANOVA was employed to assess the main and interaction effects of RT, Vit D, Ca++, Chit, and Est supplementation. Statistical analyses were performed using SPSS (version 22, IBM SPSS, Chicago, IL, USA). An a priori significance level was set to P < 0.05.
4. Results
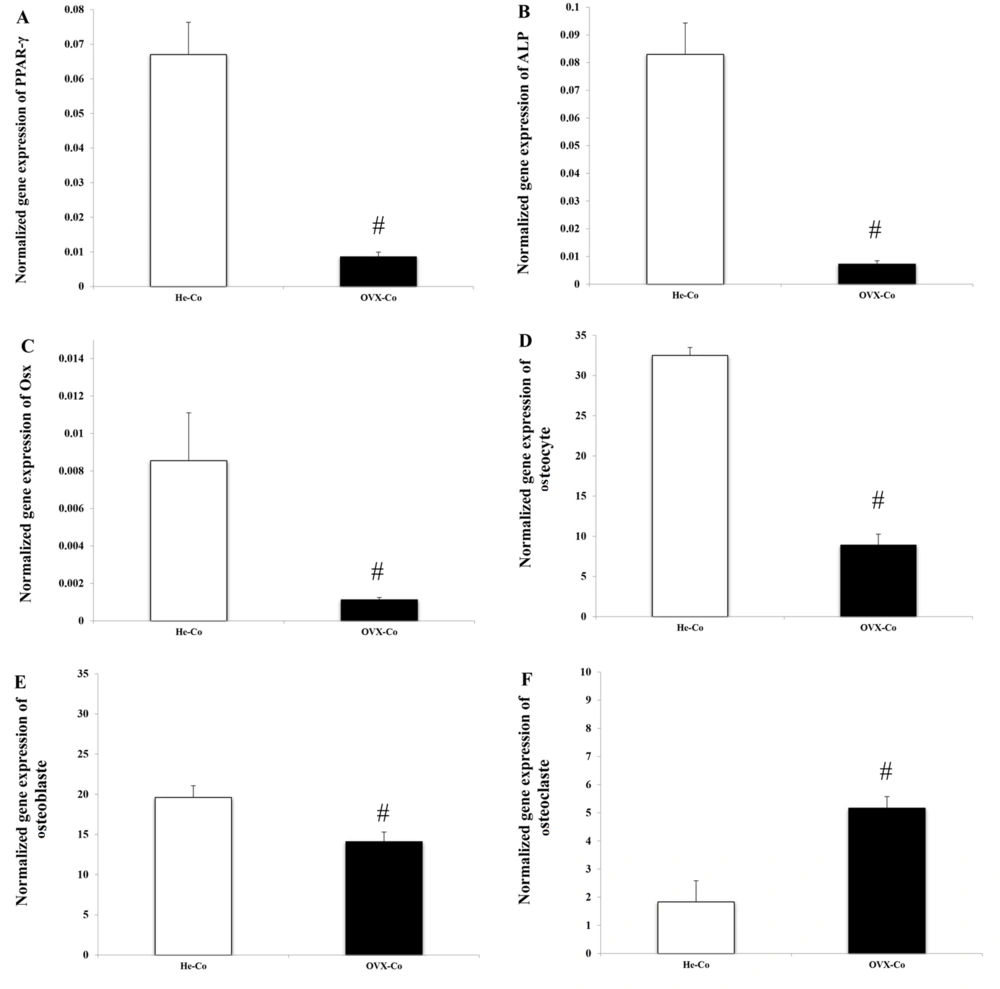
Ovariectomized induced a significant decrease in PPAR-γ (P = 0.018), ALP (P = 0.015), and Osx (P = 0.027) gene expression in bone tissue (Figure 2A, B, C). Due to menopause induction, the number of osteocytes (P = 0.001) and osteoblasts decreased, while the number of osteoclasts in bone tissue increased (P = 0.001).
Comparison of He-Co and OVX-Co: (A), PPAR-γ; (B), ALP; (C), OSX; (D), osteocytes; (E), osteoblasts; and (F), osteoclasts. Abbreviations: He-Co, healthy-control; OVX, ovariectomized; PPAR-γ, peroxisome proliferator-activated receptor gamma; ALP, alkaline phosphatase; OSX, osterix. #Signs of significant change in OVX-Co group. Information is reported based on mean ± standard deviation (SD).
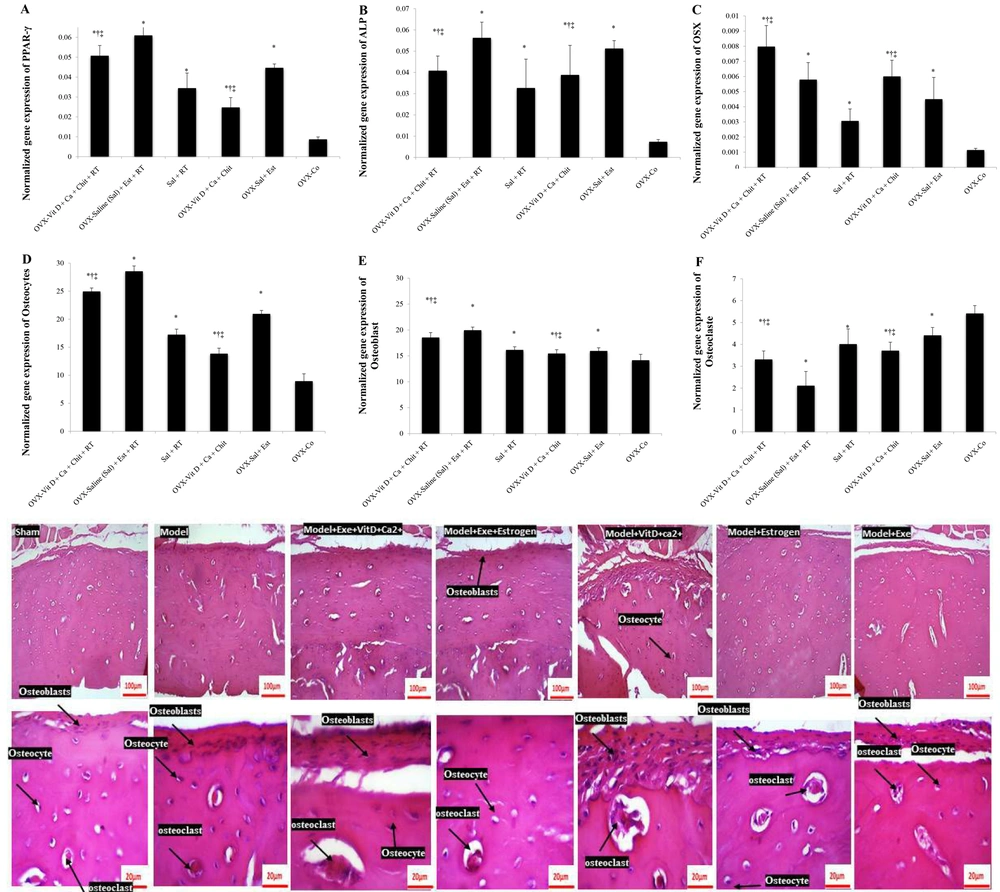
Resistance training significantly increased bone PPAR-γ gene expression (F = 73.60, P = 0.001, ƞ = 0.710). Supplementation also had a significant effect on bone PPAR-γ gene expression (F = 46.60, P = 0.001, ƞ = 0.757). At the end of the period, bone PPAR-γ gene expression was higher in the Est group compared to the Vit D + Ca++ + Chit group (P = 0.001). The expression of this gene in both the Vit D + Ca++ + Chit (P = 0.001) and Est (P = 0.001) groups was higher than in the OVX-Co group. Although the highest expression of bone PPAR-γ gene was observed in the Vit D + Ca++ + Chit + RT and Est groups, their interaction was not significant (F = 1.45, P = 0.250, ƞ = 0.088).
There was no significant difference between the Vit D + Ca++ + Chit + RT group and the Vit D + Ca++ + Chit and Est + RT groups (P = 0.500) and the Vit D + Ca++ + Chit group (P = 0.99). Peroxisome proliferator-activated receptor gamma gene expression in the Est + RT group was significantly higher than in the RT group (P = 0.001) and the OVX-Co group (P = 0.001). There was no difference between the Est + RT group and the Vit D + Ca++ + Chit group (P = 0.500). The expression of PPAR-γ gene in the RT group was lower than in the Vit D + Ca++ + Chit group (P = 0.019) but higher than in the OVX-Co group (P = 0.001). There was a difference in the expression of this gene between the RT and Est-RT groups (P = 0.019). Peroxisome proliferator-activated receptor gamma gene expression in the Vit D + Ca++ + Chit group was lower than in the Est (P = 0.002) and OVX-Co groups (P = 0.001). There was a difference in the expression of PPAR-γ gene between the Est and OVX-Co groups (P = 0.001) (Figure 3A).
Resistance training increased bone ALP gene expression (F = 12.39, P = 0.001, ƞ = 0.292). Supplementation also had a significant effect on bone ALP gene expression (F = 40.92, P = 0.001, ƞ = 0.732). At the end of the study period, bone ALP gene expression was lower in the Est group than in the Vit D + Ca++ + Chit group (P = 0.002). The expression of this gene in both the Vit D + Ca++ + Chit (P = 0.001) and Est (P = 0.001) groups was higher than in the OVX-Co group. The interaction between RT and supplementation (Vit D with Chit or Est) on ALP gene expression is significant and has an agonistic effect. The concurrence of these two interventions had an increasing effect on ALP gene expression (F = 5.71, P = 0.008, ƞ = 0.276).
Alkaline phosphatase gene expression in the exercise-Est group was different from the exercise group (P = 0.002) and the Vit D + Chit group (P = 0.038). However, the expression of this gene in the exercise-Est group was higher than in the Est (P = 1.00) and OVX-Co groups (P = 0.001). The expression of ALP gene in the exercise group was not different from the Vit D + Chit group (P = 1.00) but was different from the Est group (P = 0.022). The expression of this gene in the exercise group was higher than in the OVX-Co group (P = 0.001). Alkaline phosphatase gene expression in the Vit D + Chit group was no different from the Est group (P = 0.387) but was higher than in the OVX-Co group (P = 0.001). There was a difference in ALP gene expression between the Est and OVX-Co groups (P = 0.001) (Figure 3B).
Resistance training increased bone Osx gene expression (F = 22.25, P = 0.001, ƞ = 0.426). Supplementation also had a significant effect on bone Osx gene expression (F = 60.44, P = 0.001, ƞ = 0.801). At the end of the study period, bone Osx gene expression was higher in the Est group than in the Vit D + Chit group (P = 0.001). The expression of this gene in both the Vit D + Chit (P = 0.001) and Est (P = 0.001) groups was higher than in the OVX-Co group. Although the highest expression of bone Osx gene was observed in the combination of exercise and Vit D + Chit and exercise-Est groups, the interaction of this intervention was not significant (F = 0.35, P = 0.703, ƞ = 0.023).
Osterix gene expression in the exercise-Vit D + Chit group was different from the exercise-Est group (P = 0.026) but showed no difference with the Vit D + Chit group (P = 0.061). The expression of this gene in the exercise-Vit D + Chit group was higher than in the exercise (P = 0.001), Est (P = 0.001), and OVX-Co groups (P = 0.001). Osterix gene expression in the exercise-Est group was higher than in the exercise group (P = 0.002), but not different from the Est group (P = 0.762), and higher than in the OVX-Co group (P = 0.001). Osterix gene expression in the exercise group was lower than in the Vit D + Chit group (P = 0.001) and no different from the OVX-Co group (P = 0.076), or the Est group (P = 0.991). Osterix gene expression in the Vit D + Chit group was higher than in the Est group (P = 0.370) and the OVX-Co group (P = 0.001). Osterix gene expression in the Est group was higher than in the OVX-Co group (P = 0.001) (Figure 3C).
Resistance training increased the number of osteocytes (F = 748.97, P = 0.001, ƞ = 0.961). Supplementation also had a significant effect on the number of osteocytes (F = 419.25, P = 0.001, ƞ = 0.965). At the end of the study period, the number of osteocytes was lower in the Est group than in the Vit D + Chit group (P = 0.001). The number of osteocytes in both the Vit D + Chit (P = 0.001) and Est (P = 0.001) groups was higher than in the OVX-Co group. Exercise-supplementation interaction had a significant effect on osteocyte count (F = 10.57, P = 0.001, ƞ = 0.413), enhancing each other's effect on osteocyte counts.
The number of osteocytes in the exercise-Vit D + Chit group was different from the exercise-Est group (P = 0.001), and significant in exercise-Vit D + Chit compared to the exercise groups (P = 0.001), Vit D + Chit (P = 0.018), Est (P = 0.001), and OVX-Co group (P = 0.001). The number of osteocytes in the exercise-Est group was higher than in the Est (P = 0.001) and OVX-Co groups (P = 0.001). The number of osteocytes in the exercise group was different from the Vit D + Chit (P = 0.001) and Est groups (P = 0.001), but higher than the OVX-Co group (P = 0.001). The number of osteocytes in the Vit D + Chit group was lower than in the Est group (P = 0.001) but higher than in the OVX-Co group (P = 0.001). The number of osteocytes in the Est group was higher than in the OVX-Co group (P = 0.001) (Figure 3D).
Resistance training increased the number of osteoblasts (F = 103.25, P = 0.001, ƞ = 0.775). Supplementation also had a significant effect on the number of osteoblasts (F = 75.70, P = 0.001, ƞ = 0.835). At the end of the study period, the number of osteoblasts in the Est group was different from the Vit D + Chit group (P = 0.001). The number of osteoblasts in both the Vit D + Chit (P = 0.001) and Est (P = 0.001) groups was higher than in the OVX-Co group. The interaction between RT and supplementation (Vit D + Chit or Est) regarding the number of Osteoblasts in bone tissue was significant and has an agonistic effect, increasing the number of osteoblasts in bone tissue (F = 41.66, P = 0.001, ƞ = 0.735).
The number of osteoblasts in the exercise-Vit D + Chit group was not different from the exercise-Est group (P = 0.096). However, the number of osteoblasts increased in the exercise-Vit D + Chit group compared to the exercise groups (P = 0.001), Vit D + Chit (P = 0.001), Est (P = 0.001), and OVX-Co group (P = 0.001). The number of osteoblasts in the exercise-Est group was higher than in the Vit D + Chit group (P = 0.096), exercise group (P = 0.001), Est group (P = 0.001), and OVX-Co group (P = 0.001). The number of osteoblasts in the exercise group was lower than in the Vit D + Chit (P = 0.001) and Est groups (P = 0.001), but no different from the OVX-Co group (P = 1.000). The number of osteoblasts in the Vit D + Chit group was different from the Est group (P = 0.001) but not different from the OVX-Co group (P = 1.00). The number of osteoblasts in the Est group was higher than in the OVX-Co group (P = 0.011) (Figure 3E).
Resistance training reduced the number of osteoclasts (F = 115.20, P = 0.001, ƞ = 0.793). Supplementation also had a significant effect on the number of osteoclasts (F = 66.95, P = 0.001, ƞ = 0.817). At the end of the study period, the number of osteoclasts was different in the Est group compared to the Vit D + Chit group (P = 0.001). The number of osteoclasts in both the Vit D + Chit (P = 0.001) and Est (P = 0.001) groups was lower than in the OVX-Co group. The interaction between RT and supplementation (Vit D + Chit or Est) had an agonistic effect, increasing the number of osteoclasts (F = 25.35, P = 0.001, ƞ = 0.628).
The number of osteoclasts in the exercise-Vit D + Chit group was different from the exercise-Est (P = 0.001) and exercise (P = 0.001) groups. The number of osteoclasts was lower in the exercise-Vit D + Chit group than in the Vit D + Chit (P = 0.001), Est (P = 0.003), and OVX-Co groups (P = 0.001). The number of osteoclasts in the exercise-Est group was lower than in the Vit D + Chit (P = 0.001), exercise (P = 0.001), Est (P = 0.001), and OVX-Co groups (P = 0.001). The number of osteoclasts in the exercise group was different from the Vit D + Chit (P = 0.001) and Est groups (P = 0.023), but no different from the OVX-Co group (P = 1.00). The number of osteoclasts in the Vit D + Chit group was not different from the Est group (P = 1.000), but higher than the OVX-Co group (P = 0.001). The number of osteoclasts in the Est group was higher than in the OVX-Co group (P = 0.001) (Figure 3F).
(A), PPAR-γ; (B), ALP; (C), OSX; (D), osteocytes; (E), osteoblasts; and (F), osteoclasts changes in OVX groups. Abbreviations: OVX, ovariectomized; PPAR-γ, peroxisome proliferator-activated receptor gamma; ALP, alkaline phosphatase; OSX, osterix; Sal, saline; RT, resistance training; Est, estrogen; Vit D, vitamin D; Chit, chitosan; Ca++, calcium. *Signs of a significant difference to the OVX-Co. †Signs of a significant difference to the Sal + RT group. ‡Signs of a significant difference to the Sal+ Est group. Information is reported based on mean ± standard deviation (SD).
5. Discussion
The major findings demonstrated that induced menopause decreased bone tissue expression of ALP and OSX while PPAR-γ gene expression increased. Ovariectomized also decreased the number of osteocytes and osteoblasts while increasing osteoclast cells. These results indicated the negative effects of OVX on bone. Comparing He-Co and OVX-Co demonstrated that removing the ovaries resulted in menopause, suggesting that the other OVX groups experienced a similar loss. During menopause, bone loss was significantly accelerated, increasing the likelihood of osteoporosis. The drop in Est levels around menopause results in increased bone loss. Established evidence shows that Est receptors regulate osteoblast and osteoclast functions (26-28). Other mechanisms relate to changes in several genes. Bone ALP plays a crucial role in regulating bone mineralization by hydrolyzing inorganic pyrophosphate, which naturally inhibits mineralization (29). The increase in serum ALP levels observed post-menopause is attributed to elevated bone turnover (30). Bone ALP is anchored to the outer surface of osteoblasts through membrane inositol-phosphate. The expression of the transcription factor OSX is necessary for osteoblast differentiation (31). Variants in PPARD and PPARGC1A genes have been linked to menopause (32).
Furthermore, additional findings from the current study demonstrated that RT increased bone tissue expression of ALP and OSX and increased PPAR-γ gene expression. It also increased the number of osteocytes and osteoblasts while decreasing osteoclast cells. The effect of physical activity on bone turnover and related genes has been investigated by several studies (33-35). According to Marini et al., exercise may benefit osteoporotic individuals by decreasing bone resorption biomarkers and improving bone formation (34). Roghani et al. demonstrated that a brief period of submaximal walking while wearing a weighted vest can effectively stimulate bone formation (as indicated by increased ALP) and reduce bone resorption in postmenopausal women with osteoporosis (35). Singulani et al. showed that strength training increased OSX gene expression and reduced PPARγ expression (36). Mechanical loading down-regulates PPARγ in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis (37).
Previous studies have shown that nutritional compounds positively affect molecular structure and have beneficial effects on human diseases (38-40). The current study demonstrated that Vit D-Chit supplementation led to an increase in ALP and OSX gene expression in bone tissue, along with an increase in PPAR-γ gene expression. Additionally, it resulted in an increased number of osteocytes and osteoblasts while reducing the number of osteoclast cells. Limited information is available regarding the impact of Vit D-Chit supplementation on PPARγ, OSX, ALP, osteoblast, and osteoclast factors specifically in menopausal conditions. Researchers have demonstrated that Vit D enhances the expression of PPAR-γ and augments the beneficial effects of physical activity in individuals with metabolic syndrome (17). Chitosan, a fibrous material from shrimp, crabs, and mushrooms, has been shown to help reduce menopausal symptoms and alter gut microbiota in rats lacking Est (18, 19). Acetylated Chit oligosaccharides inhibit glutamate-induced death in PC12 cells (20). Chitosan enhances the activity of certain genes when bone marrow stem cells turn into bone cells (21); however, there is limited literature on PPARγ and OSX genes. Previous studies have demonstrated that the delivery of PPAR using Ch-GNPs on titanium surfaces effectively suppressed inflammation caused by implants and stimulated osteoblast-like cells to promote bone mineralization (41).
5.1. Conclusions
In conclusion, the present study showed that OVX decreased bone tissue expression of ALP and OSX while increasing PPAR-γ gene expression. Ovariectomized also decreased the number of osteocytes and osteoblasts while increasing the number of osteoclasts. However, RT and Vit D + Ca++ + Chit supplementation reduced the harmful effects of OVX. The combined RT + Vit D + Ca++ + Chit and Est replacement therapy had a synergistic effect compared to each intervention alone on ALP expression, and osteocyte, osteoblast, and osteoclast cell numbers. These findings suggest that future researchers should consider a combined intervention approach (RT, supplementation, and Est replacement therapy) for menopausal and post-menopausal women to determine if a similar synergistic effect could be realized.