1. Background
Breast cancer significantly impacts women globally, demonstrating an increasing incidence in both developed and developing countries (1). According to the new statistics in Iran, 6160 breast malignancies are diagnosed in the country each year, and 1063 cases cause death (2). Despite the limited knowledge of the actual etiology of breast cancer, numerous studies have yielded compelling data indicating that the disease is likely attributable to a multifaceted interplay of various factors. This sickness demonstrates associations with several risk variables, such as genetic modifications, dietary patterns, nursing practices, and environmental influences (3). The main determinant linked to breast cancer is the existence of genetic anomalies in crucial genes, including tumor suppressor genes, growth-regulating genes, and oncogenes (4). Furthermore, prior research demonstrated that certain variations in a single nucleotide, known as single nucleotide polymorphisms (SNPs), can have a detrimental impact on the range of observable characteristics in individuals. These SNPs have been found to be linked to a heightened susceptibility to cancer and disease progression (5, 6).
The genetic elements responsible for encoding hemoproteins, specifically cytochrome P450 (CYPs), have been extensively involved in several metabolic and clearance mechanisms (7). Moreover, it is essential to acknowledge that these genes substantially impact drug metabolism. Several CYP genes have been associated with the initiation and progression of cancer as a result of their involvement in the acceleration of oxidative stress, activation of procarcinogens, and inactivation of anticancer medications (8, 9). The existence of hereditary genetic variants at both the individual and community levels has a role in the development of interethnic disparities in cancer susceptibility and treatment outcomes (10). During this inquiry, an extensive analysis will be conducted on a set of genes, specifically CYP19A1, CYP2C19, CYP2C9, CYP1B1, CYP3A4, and CYP1A2.
Emphasis on the aromatase enzyme, generated by the CYP19A1 gene, stems from its notable role in estrogen biosynthesis (11). Therefore, aromatase inhibitors (AI) are employed for this purpose. Previous studies investigated the influence of CYP19A1 polymorphisms on various factors, including estrogen levels in the bloodstream, tumor characteristics, the occurrence of arthralgia associated with AI usage, the decline in bone density linked to AI usage, and the effectiveness of letrozole in breast cancer patients (12, 13). Similarly, it is important to highlight that the CYP19A gene is responsible for generating a liver enzyme primarily recognized for its role in the metabolism of the antiplatelet medication clopidogrel. It is imperative to highlight that genetic variations within this specific gene can potentially generate inconsistent therapeutic outcomes (14). The findings of a study indicate a minimal statistical impact of CYP19A polymorphisms on the response of Asian breast cancer patients to tamoxifen therapy. The present discovery contradicts the established association between a distinct polymorphism and a reduced occurrence of breast cancer in individuals of Caucasian ancestry (15, 16). In addition, it is crucial to recognize that the CYP2C9 gene plays a substantial role in phase I metabolism. However, due to its highly diverse characteristics, there are discrepancies in metabolic activity and the likelihood of adverse medication reactions (17). Empirical evidence suggests a probable association between genetic variations in the CYP2C9 gene and both disease-free survival rates and tumor features in breast cancer patients receiving tamoxifen treatment. However, the literature has shown divergent findings (15, 18).
The precise association between genetic alterations in the CYP1A2 gene and breast cancer initiation has yet to be established.
2. Objectives
The primary aim of this study was to examine the potential correlation between the rs17861162 polymorphism of the CYP1A2 gene and susceptibility to breast cancer in women of Iranian descent.
3. Methods
3.1. Patients and Sample Collection
In this case-control study, a cohort including 200 female participants was purposefully chosen from educational institutions in Tabriz, Iran, from December 2021 to January 2023. The research was conducted exclusively with female participants aged 40 - 60 years. The current study utilized a cohort of 100 participants who received a diagnosis of breast cancer through physical examinations, histological investigations, and imaging assessments. Moreover, a control group, including 100 women with perfect health, similar age and ethnicity, and no familial susceptibility to cancer, was selected to enable regular medical evaluations. After collecting the samples, all participants abstained from consuming anti-inflammatory medication for 72 hours. In addition, to mitigate potential confounding factors, the study excluded female individuals with pre-existing medical illnesses, including kidney, cardiovascular, metabolic, or hepatic disorders. Data pertaining to clinical features, lifestyle factors, and demographic information was collected by implementing interviews and administering questionnaires to both the case and control groups. The variables examined in this study consisted of age (year), body mass index (BMI kg/m2), age at menarche (year), menopausal status, tobacco smoking, alcohol consumption, age at first delivery (years), and family history of breast cancer. In order to address any epidemiological bias, the study participants were intentionally selected exclusively from the East Azerbaijan region of Iran. Considerable attention was devoted to ensuring that the chosen female volunteers were carefully matched based on age and ethnicity and had no genetic relatedness. Before initiating the investigation, all subjects were presented with extensive details about the study. They were requested to grant their consent by affixing their signature to a document, per the ethical principles outlined in the Helsinki Declaration.
3.2. DNA Extraction and Genotyping
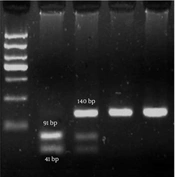
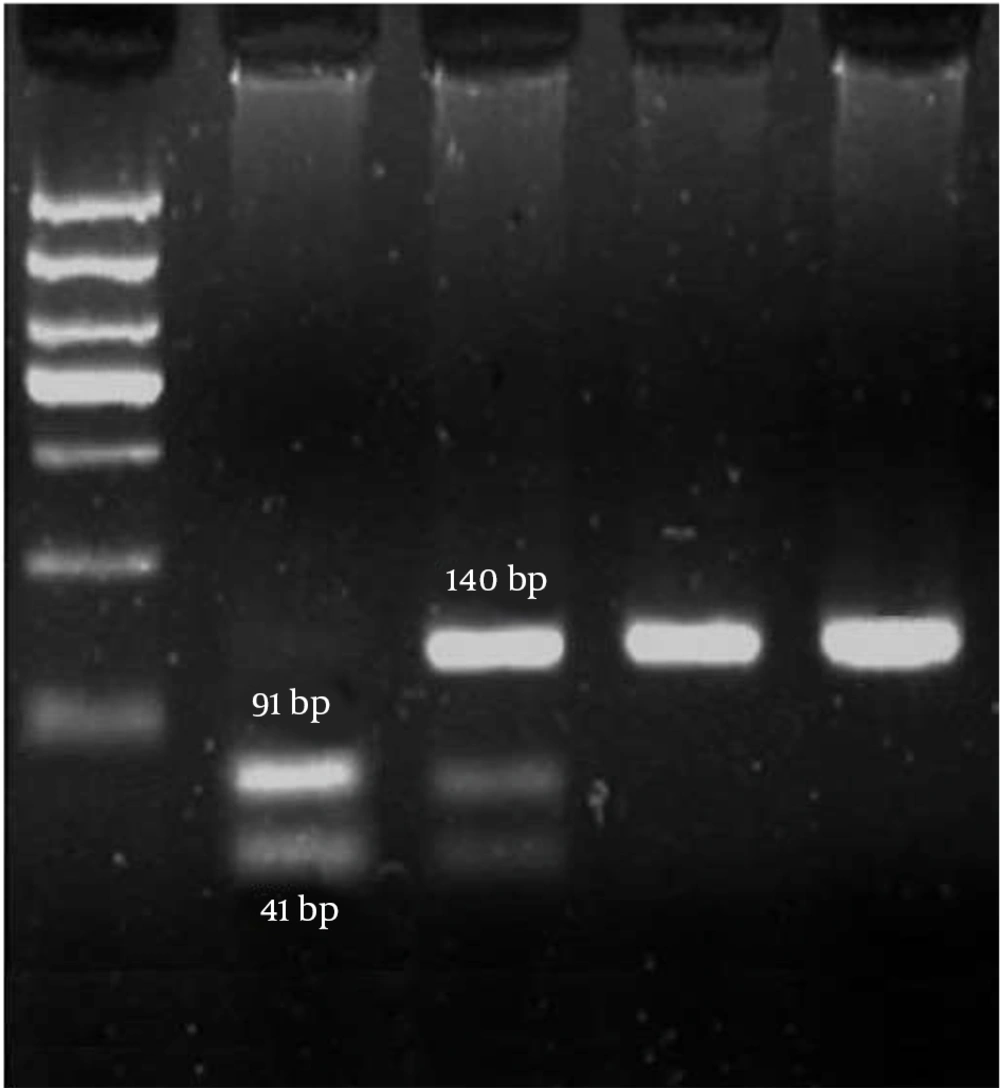
A blood sample of 5 mL was collected from each participant using vials pre-treated with the anticoagulant ethylenediamine diamine tetraacetic acid. The genomic DNA was extracted from the peripheral blood leukocytes using the phenol-chloroform technique. The OD 260/280 ratio was utilized to evaluate the concentration and quality of the isolated DNA by a nanodrop instrument. A ratio of 1.7 to 1.9 was preferred. Furthermore, the findings were verified by electrophoresis on a 1% agarose gel. The DNA samples were maintained at a temperature of -20°C prior to the genotyping procedure. The genotyping experiment was conducted as polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The primer sequences utilized for the forward and reverse strands were 5′-GCCTCGACCTCTCTCTCAAAGT and 5′-GCAGGCTAGGGGGAAATGT, respectively. The PCR products, with 140 base pairs (bp), underwent digestion using the BSP12861 restriction enzymes at 37° C. When the C allele was present, the PCR product underwent enzymatic digestion, producing two fragments of 91 and 49 bp lengths. In contrast, the presence of the G allele did not modify the PCR outcome. The rs17861162 polymorphism displayed discernible patterns of RFLP, including CC (91 bp + 49 bp), CG (140 bp + 91 bp + 49 bp), and GG (91 bp + 49 bp). The PCR reaction was carried out in a total volume of 25 μL, including forward primer (25 pmol), reverse primer (25 pmol), template DNA (1 μg), dNTP (0.1 mmol), PCR buffer (2.5 μL), MgCl2 (1.5 mmol/L), and Taq DNA polymerase (1.5 unit) in the following condition: initial denaturation (1 cycle in 95°C for 5 min), denaturation (30 cycles in 95°C for 1 min), annealing (30 cycles in 55°C for 1 min), extension (30 cycles in 72°C for 1 min), and final extension (1 cycle in 72°C for 5 min). The digested fragments were separated using electrophoresis on 3% agarose gel stained by ethidium bromide. A 50 bp marker (ladder) was used to estimate the size of DNA bands. Finally, a gel documentation instrument was used to visualize the bands of digested PCR products.
3.3. Statistical Analysis
The collected data were statistically analyzed using the Statistical Package for the Social Sciences (SPSS) version 21.0. We used logistic regression analysis to examine the correlation between the rs17861162 polymorphism and the probability of developing breast cancer. The Chi-square (χ2) test and Fisher’s exact test were employed to evaluate the conformity of genotype distribution in individuals with breast cancer and healthy individuals to the principles of Hardy-Weinberg equilibrium. Furthermore, we assessed the odds ratio (OR) and 95% confidence intervals (CI). We employed an independent samples t-test to examine the disparities in demographic and clinical attributes between individuals diagnosed with breast cancer and those in a healthy condition. A significance level of 0.05 was determined for statistical analysis.
4. Results
In order to investigate the correlation between the rs17861162 polymorphism of the CYP1A2 gene and breast cancer in the Iranian population, a cohort consisting of 100 individuals diagnosed with breast cancer (case group) and 100 healthy women of similar age and ethnicity (control group) were deliberately selected from the East Azerbaijan province.
Table 1 displays the genotype and allele frequency distribution for the rs17861162 polymorphism in both the case and control groups. Two separate investigations have reported the identification of the rs8661162 polymorphism in both case and control groups, resulting in P > 0.05.
| Genotype and Allele | Patients (N=100) | Controls (N=100) | P-Value | OR (95% CI) |
|---|---|---|---|---|
| CC | 54 (54) | 69 (69) | Ref | Ref = 1 |
| CG | 37 (37) | 29 (29) | 0.026 | 1.34 (1.10 - 1.64) |
| GG | 9 (9) | 2 (2) | 0.022 | 0.70 (0.52 - 0.93) |
| C normal | 145 (72.5) | 167 (83.5) | Ref | Ref = 1 |
| G mutant | 55 (27.5) | 33 (16.5) | 0.008 | 1.92 (1.18 - 3.11) |
Abbreviations: OR, odds ratio; CI, confidence interval.
a Statistically significant P < 0.05.
According to the findings, the group of patients exhibited a prevalence of 54% for the homozygous CC genotype, 37% for the heterozygous CG genotype, and 9% for the homozygous GG genotype. In addition, the group of patients demonstrated a prevalence of 69% for individuals with homozygous CC genotypes, 29% for heterozygous CG genotypes, and 2% for homozygous GG genotypes. The rs17861162 polymorphism exhibited a statistically significant disparity (P < 0.05) in the genotype frequencies observed between those diagnosed with breast cancer and those unaffected by the aforementioned ailment. The electrophoresis data presented in Figure 1 were employed for genotyping the rs17861162 polymorphism.
Based on the results shown in Table 1, it is evident that the prevalence of the C allele was 72.5% within the patient cohort, while in the control group, including persons categorized as healthy, the prevalence of the C allele was 83.3%. Furthermore, it was shown that the prevalence of the G allele was 27.5% within the affected population, whereas it was 16.5% in the unaffected individuals in the control cohort. The analysis of allele frequencies demonstrated a statistically significant disparity (P < 0.05) between the groups of individuals affected by the investigated illness and those who were not affected.
5. Discussion
Extensive study has investigated numerous agents and genetic risk factors to explore the intricate, diversified, and multifaceted aspects of breast cancer (5, 6). Previous studies demonstrated that genetic variants in the CYP genes can impact both the susceptibility and progression of breast cancer, as well as an individual’s pharmacological response to anticancer medications (19, 20). This study aimed to assess the correlation between the genetic variant rs17861162 and the susceptibility to breast cancer in women of Iranian ancestry. The current investigation, comprising a cohort of 100 individuals diagnosed with breast cancer and 100 healthy individuals, unveiled a significant correlation between the rs17861162 polymorphism and susceptibility to breast cancer among women in Iran.
The enzyme CYP1A2 is of significant importance in facilitating the metabolic pathways of various endogenous substrates, including retinoic and bile acids, as well as the steroid hormones testosterone and estrogen (21). A positive link has been observed between the risk of breast cancer in postmenopausal women and estrogen levels, which are impacted by both menstrual status and the age of the patient (22). A comprehensive meta-analysis comprising 46 case-control studies revealed a statistically significant association between the CYP1A2* 1F polymorphism and vulnerability to estrogen-related breast and ovarian cancers. However, another study showed no relationship between various types of cancer, including lung, colorectal, bladder, endometrial, pancreatic, and gastric cancers (23). Our findings indicated a potential association between genetic polymorphisms in the CYP1A2 gene and the ability to induce and the efficacy of the enzyme. The association between this linkage can give rise to metabolic abnormalities linked to estrogen and progesterone, finally culminating in an augmented susceptibility to breast cancer (24). We found that three SNPs located in the CYP1A2 locus can potentially modify the activity of CYP1A2, an enzyme that plays a critical role in regulating estrogen metabolism. Therefore, it is conceivable that these genetic variations may potentially impact the susceptibility of females to breast cancer among different age groups. One of the identified SNPs, specifically referred to as rs17861162, is located inside the 3’-UTR of the CYP1A2 gene. A previous study reported that this polymorphism is associated with the dose of epidural ropivacaine in patients undergoing breast cancer surgery (25). Another investigation (26) demonstrated a significant association between this particular polymorphism and the administration dosage of epidural ropivacaine in breast cancer patients. The results of this study suggest a statistically significant association between the identified genetic variant and the likelihood of developing breast cancer. Vukovic et al. (27) did not reveal a statistically significant association between the rs17861162 polymorphism and breast cancer. Furthermore, an investigation by Bai et al. (20) failed to establish any validated correlation between the rs17861162 polymorphism and the probability of developing breast cancer. The discrepancies identified in various investigations can be ascribed to the existence of interconnected genes, environmental factors, fluctuations in sample size, ethnic composition, racial heterogeneity, and geographic distribution (28-30).
5.1. Conclusions
This study presented empirical findings that highlight the complex characteristics of breast cancer and suggest a potential correlation between the existence of the CYP1A2 gene rs17861162 variant and increased vulnerability to breast cancer in Iranian women. However, there remains a remarkable gap in knowledge regarding the precise function and consequences of the rs17861162 polymorphism in the CYP1A2 gene concerning breast cancer. In order to enhance the comprehension of the correlation between this specific polymorphism and breast cancer, it is advisable to conduct additional research endeavors that involve larger sample sizes and span a wide range of racial and ethnic backgrounds.