1. Background
Statistical studies by the World Health Organization (WHO) in 185 countries show that cancer is the first or second cause of death in people under the age of 70 in most of these countries (1). In the meantime, lung cancer ranks second after breast cancer, with a frequency of 11.4%. In addition, lung cancer is the most fatal type of cancer, with 1.8 million deaths per year (18%) (1). Statistics show that the rate of lung cancer is 3 to 4 times higher in developing countries than in developed countries, and smoking is responsible for about two-thirds of the cases (2). The 5-year survival rate in lung cancer patients is reported to be 10 - 20% (3).
Lung cancer is in the group of heterogeneous cancers and is divided into different classes based on histological characteristics. The most common of these groups are small cell lung cancer and non-small cell lung cancer (NSCLC) (4). Immunohistochemical examination, genetic changes, and diagnostic biomarkers are necessary to confirm non-small cell lung cancer. Studies show that the expression of the EGFR gene increases in different types of lung cancer, indicating the importance of this gene and its related pathways in this cancer (5, 6).
Non-small cell lung cancer has the highest incidence rate, with a frequency of 80 - 85%. Molecular studies show that 40% of Asian and 20% of non-Asian patients have mutations in the EGFR gene (7-9). For this reason, the first and second-generation drugs for treating this disease are designed to affect this gene (10-17). The EGFR gene, also known as ERBB or HER1, encodes a transmembrane protein belonging to the superfamily of protein kinases. This protein leads to cell growth by receiving epidermal growth factor and through the mechanism of dimerization and autophosphorylation (18). Dysfunction of this gene is attributed to lung cancer and cytokine storm involved in coronavirus (19, 20). The EGFR gene, with chromosomal location 7p11.2 and 32 exons, has the most mutations related to lung cancer in exons 19 and 21 (18). Most of the known mutations in these exons have been of the deletion or substitution type (21).
Despite the important role of this gene in the diagnosis and treatment of lung cancer, no comprehensive studies have been conducted on the common mutations of this gene in the Middle East and Iran.
2. Objectives
In this study, by examining 20 paraffin blocks belonging to patients with different types of NSCLC, we sought to determine the common mutations in Iranian patients so that we could help to complete the EGFR gene mutation map at the international level. In addition, with the data obtained from this research and knowing as much as possible about the mutations of the Iranian population and comparing it with common mutations in the world, it is possible to help with faster screening and person-based drug therapy.
3. Methods
3.1. Preparation of Samples
In this cross-sectional case study, 20 blocks of NSCLC patients referred to Tehran Cancer Institute between 2020 and 2023 were prepared. The diagnosis of these patients was made using small biopsies and large sampling (transbronchial, endobronchial biopsies, CT-guided central biopsies, or mass sampling). The samples included 5 women and 15 men aged 61 - 74.
3.2. Preparing the Block for Purification
The excess paraffin was cut from the sample block using a knife, and a thickness of 5 - 10 micrometers was separated from the tumor areas and normal tissue of each patient with the help of a surgical blade. Then, it was cut into thin slices and placed into a sterile 1.5 mL microtube with tweezers. In order to remove paraffin, the samples were washed three times with pure xylene. For this purpose, 1 mL (1000 microliters) of pure xylene was added, vortexed for 10 seconds, and centrifuged at room temperature at 14000 rpm for 2 minutes. At this stage, a precipitate was formed, and then the supernatant was carefully separated by pipetting. This process was repeated three times until the paraffin was completely removed. In the next step, to remove xylene twice, 1 mL (1000 microliters) of cold ethanol (100%) was added to the tube containing the tissue and centrifuged for 2 minutes under the same conditions as in the previous step. Then, the alcohol was carefully separated and removed with a pipette. The samples were placed for 15 minutes at a temperature of 37°C to completely remove ethanol in the tissues (some samples took more time to dry completely). At this stage, to remove alcohol faster, one drop of acetone could be added to the tissues, but in this research, it was not added. The 2 × 20 μm paraffin blocks were used for DNA extraction (22). The QIAamp DNA FFPE tissue kit (QIAGEN) (Cat. No: 56404) was used for DNA extraction according to its protocol.
3.3. Real-time Polymerase Chain Reaction
The RT-PCR was performed using a Rotor-Gene Q machine (USA). The design of primers and probes was done using Oligo7 software (Table 1). To ensure the specific function of the primers, the designed sequences were BLASTed on the NCBI website. Based on the information about each primer, a specific volume of DEPC water was added, and finally, the concentration of all primers reached 100 pmol and was kept at -20°C. The primers used to detect the deletion in exon 20 of the EGFR gene were designed by two reverse primers, whose positions are marked with green color. Two probes are located at the T790M mutation site (C > T). One probe detects the normal allele, while the other probe specifically detects the mutant allele. Both probes are located in the coding region of the exon.
| Exon No. | Primer Sequence | Probe Sequence |
|---|---|---|
| Exon 20 | FORWARD 5-CTCTCCCTCCCTCCAGGAAGCC-3 | PROBE (ALLELE NORMAL): 5-CTCCACCGTGCAGCTCATCAC-3 |
| REVERSE 5-GTGTTCCCGGACATAGTCCAG-3 | PROBE (ALLELE MUTANT): 5-CTCCACCGTGCAGCTCATCAT-3 | |
| Exon 21 | FORWARD 5-CCTCCACCGTGCAHCTCATCAT-3 | PROBE (ALLELE NORMAL): 5-GCCCGACATTCTGCAAGTCC-3 |
| FORWARD 5-CCGTATCTCCCTTCCCTGATT-3 | PROBE (ALLELE MUTANT): 5-GCCCGACATTCTGCAAGTCA-3 |
Primer Sequence and Probes Used in the Research
The relative expression levels of DNA were calculated using Ct values, and the normalized expression level of the target genes was compared to the reference genes. Finally, the gene expression data were compared using the Livak (2-ΔCT) method. The sequences of primers and probes are given in Table 2. The Real-time PCR was used with the following temperature program for both genes: 15 min at 95°C followed by 40 cycles of 30 s at 95°C, 34 s at 60°C, and 30 s at 72°C. Melting curve analysis and gel electrophoresis of the products were performed to check the specificity of the primers.
| Tissue | Age | Gender | Smoker | Family History | Mutation | |
|---|---|---|---|---|---|---|
| 1 | Squamous cell carcinoma | 66 | Male | Yes | No | Negative |
| 2 | Adenocarcinoma | 71 | Male | Yes | No | Negative |
| 3 | Large cell carcinoma | 74 | Male | No | No | Negative |
| 4 | Squamous cell carcinoma | 62 | Female | Yes | Yes | Negative |
| 5 | Squamous cell carcinoma | 76 | Female | Yes | Yes | Negative |
| 6 | Large cell carcinoma | 63 | Male | Yes | Yes | L861Q |
| 7 | Adenocarcinoma | 65 | Male | No | Yes | Negative |
| 8 | Large cell carcinoma | 69 | Male | No | No | Negative |
| 9 | Adenocarcinoma | 72 | Male | No | Yes | Negative |
| 10 | Large cell carcinoma | 70 | Female | No | Yes | Negative |
| 11 | Adenocarcinoma | 71 | Male | Yes | No | Negative |
| 12 | Large cell carcinoma | 71 | Male | No | No | Negative |
| 13 | Large cell carcinoma | 68 | Male | No | No | Negative |
| 14 | Large cell carcinoma | 69 | Male | No | No | Negative |
| 15 | Squamous cell carcinoma | 67 | Female | Yes | Yes | L858R |
| 16 | Squamous cell carcinoma | 66 | Male | No | Yes | Negative |
| 17 | Squamous cell carcinoma | 66 | Male | Yes | No | Negative |
| 18 | Large cell carcinoma | 73 | Female | Yes | Yes | Negative |
| 19 | Adenocarcinoma | 71 | Male | No | No | Negative |
| 20 | Large cell carcinoma | 70 | Male | No | No | Negative |
Patient Information
GraphPad version 8 statistical software was used for data analysis. The Mann-Whitney test was used to compare DNA expression between patients and controls. Data are expressed as SEM. The P-values less than 5% were considered statistically significant.
4. Results
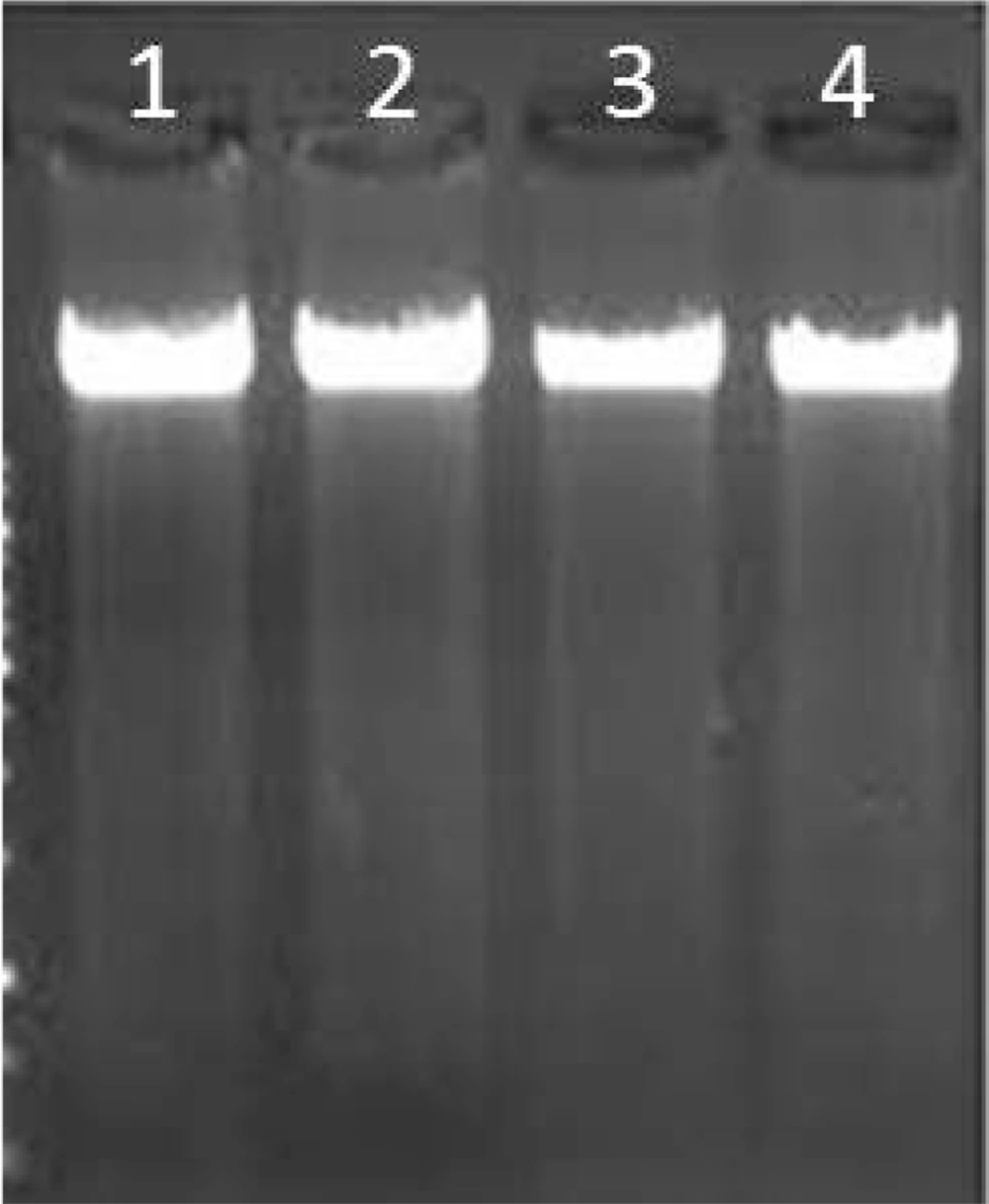
All studied blocks were examined and evaluated by a pathologist. The investigated tumors were of squamous cell type in six cases, adenocarcinoma in five cases, and large cell origin in nine cases. Clinical information related to the blocks is given in Table 2. The quality of extracted genomic DNA was checked by electrophoresis (Figure 1). The ratio of 260/280 in the extracted samples, which falls within the range of 1.8 to 2, indicates the high purity of the extracted DNA. Of course, the clear and dense bands indicate a high concentration of DNA. Therefore, the results obtained at this stage indicated that the extracted DNAs could be used with high confidence in the next stages of this research.
The total number of examined patients was 20, of whom 3 had mutations in exon 20 and 2 had mutations in exon 21, so the frequency of mutations in this group was 15% and 10%, respectively. Based on the obtained results, there was no significant relationship between the type of malignancy and being a smoker or non-smoker because the significance level was more than 0.05. Tukey's test was performed to confirm that there was no significant difference between these groups (Tables 3 and 4). Based on the results obtained from Tukey's test, all the averages were grouped into one group. This indicates that there was no significant relationship between the test groups (lung malignancies) and smoking (P = 0.075).
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Variance between groups | 1.361 | 2 | 0.681 | 3.244 | 0.065 |
| Variance within groups | 3.589 | 17 | 0.211 | ||
| Total | 4.950 | 19 |
Analysis of Variance Results Based on Smoking
| Type of Malignancy of the Mass | N | Subset for Alpha = 0.05 |
|---|---|---|
| Squamous cell carcinoma | 6 | 1.667 |
| Adenocarcinoma | 5 | 1.600 |
| Giant cell | 9 | 1.7778 |
| Significance level | 0.075 |
Tukey's Test Results
Out of the total number of examined patients, 9 people, i.e., 45%, had a family history, and 55% of the patients had no family history. Out of 9 people who had a history of the disease in their relatives, 2 had a mutation in this exon, so 22.22% had a mutation in exon 21 with a family history.
The chi-square value obtained from the comparison of group frequencies in different categories of age was 3.52 and not statistically significant (P = 0.171). Therefore, in the experimental group, mutation type and age had no significant difference.
5. Discussion
Today, according to global statistics, lung cancer is the most common and fatal type of cancer regardless of gender. This has caused the death rate due to lung cancer to rise sharply in recent years and impose various costs on societies (23). Also, 90% of lung cancers are caused by long-term exposure to secondhand smoke. The percentage of lung cancer in people who do not smoke is 15% (24). Small cell lung cancer (SCLC) accounts for about 20% of lung cancers, and NSLC accounts for about 80%. Having information about EGFR mutations is highly beneficial in predicting the response of cancer patients to treatment. Consequently, it has garnered significant attention from researchers in recent decades (25).
The research conducted in Iran to identify pathogenic mutations in the EGFR gene has so far been mostly conducted on lung cancer tumors, including NSCLC and SCLC. One of the new aspects of the current research is the targeted examination of adenocarcinoma in patients with NSCLC. Another point is the use of techniques that, in previous reports, did not have enough sensitivity to identify mutations in a small number of cancer cells. One of the commonly used techniques is sequencing or reverse dot blotting, both of which have low sensitivity. In Iran, the most common mutations in the EGFR gene have not yet been performed with the real-time method (TaqMan). This method can identify mutations in tumor tissues with less than 15% cancer cells. It is expected that due to the high sensitivity and accuracy in detecting mutations, targeted treatment can be proposed for Iranian patients with EGFR-type lung cancer.
Race, lifestyle, and exposure to carcinogens can have different effects on creating the frequency pattern of these mutations. The EGFR gene and its family play a role in all types of cancers, so it can be used to help with treatment goals and disease prognosis (26). Mutations in this gene have been seen in all types of cancers, such as head and neck, colorectal, and breast cancer, but it is more common in NSCLC lung cancer (27).
The study of Alhashimi et al. showed the change in the expression pattern of other genes, such as dcc and cdh1 genes, in gastric cancer (28). Besides, 90% of mutations are located in codons 746 to 750 of exon 19 and codon 858 of exon 21 (29). In this research, we specifically examined codon 858 and, in addition to codon 861.
In 2018, Zhang et al. reported the mutation rate in the EGFR gene in exon 21 to be 43% of all EGFR gene mutations (30). This frequency percentage does not mean the presence of 41% of mutations in exon 21, but it means that among the total mutations found, which was about 11%, 41% were in exon 21. Samples from 20 patients showed that 15% of patients had mutations in exon 20 of the EGFR gene. The T790M mutation was observed in the 11th case of adenocarcinoma and the third case of large cell cancer. In the interpretation of this difference, it should be noted that the diagnosis of the histopathological type of malignancy in the present study (in all the studied samples) was performed by biopsy with bronchoscopy. Since squamous cell carcinoma and small cell carcinoma present as central masses with intrabronchial growth, while adenocarcinomas and large cell carcinomas are typically observed as nodules or peripheral masses with pleural involvement, it can be expected that larger cell cancers will be more frequently detected in samples obtained from bronchoscopy.
In the present study, the most common type of lung malignancy in both smoking and non-smoking groups was large cell cancer. It is noteworthy that in other studies carried out in the country, the most common type of malignancy is large cell cancer, followed by adenocarcinoma or squamous cell carcinoma.
As mentioned, smoking is one of the most important causes of lung cancer. Although smoking increases the risk of all types of lung malignancies, smoking increases the risk of squamous cell cancer in men and the risk of adenocarcinoma and large cells in women. In the current study, the high rate of smoking in the group with large cell cancer, the higher average age of this group, and the increase in smoking with age are all in favor of the hypothesis that smoking plays a greater role in causing large cell cancer.
Comparing the results of this research with findings from studies conducted in other regions of the world, it is evident that the frequency of mutation in exon 21 of the EGFR gene in Iranian patients is not very similar to that of East Asian patients but rather that of Western European and Northern European patients. Although there has been limited research on the frequency of this mutation among patients from Middle Eastern countries, comparing this research with limited previous research, we can conclude that the frequency of mutation in exon 21 of Iranian patients is close to its frequency in Middle Eastern patients.
Therefore, Middle Eastern patients and European patients have the same frequency as Iranian patients in the mutation in exon 21 of the EGFR gene. Although it was expected that Iranian patients would be more similar to East Asian patients due to their closer geographical proximity, this study, consistent with similar studies, concluded that Iranian patients exhibit a high degree of similarity to European patients in terms of mutation frequency. Due to the involvement of environmental and genetic factors in the mutation of this gene, it can be said that the lifestyle and diet of Iranian people are more similar to the people of Western Europe and Northern Europe. Probably, the similarity in lifestyle, as well as the close gene pool and genetic similarity between the people of the Middle East and Iran, compared to the people of East Asia, was likely the reason for the contrasting results obtained in these regions.
5.1. Conclusions
The results showed that of 20 patients, 15% had a mutation in exon 20 of the EGFR gene. The T790M mutation was observed in the 11th and 19th patients of the adenocarcinoma type and the third patient of the large cell cancer type, which can be a confirmation of this result. We hypothesize that the most pathogenic mutations in NSCLC lung cancer patients are in exon 20 of the EGFR gene. In this research, among the 20 patients examined, two were found to have mutations in exon 21. Therefore, 10% of the patients had mutations in exon 21. Since one of the mutations found was L858R and one was L861Q, the frequency of each of the mentioned mutations in exon 21 was 5%.