1. Background
The skin is a complex tissue and the largest organ of the body, which is, in order from the inside to the outside, the dermis layer with a complex structure including fibroblast cells, extracellular matrix, nerve cells, lymphatic and blood vessels, the basement membrane, which is at the lowest level of the epidermis layer, and the epidermal keratinocyte layer (the first defense barrier of the body that separates the internal environment from the external environment) has hair follicles and sweat glands (1).
A wound is a kind of physical damage that leads to a disturbance in the body's natural structure. In this type of damage, the skin's structure can be severely damaged (2).
Damage to the tissue leads to the initiation of the body's natural physiological responses, which ultimately leads to wound healing. Treatment methods such as using common chemical drugs and medicinal plants help accelerate this process. Treatment methods such as the use of ultrasound waves and electromagnetic waves are also introduced as auxiliary or alternative treatments (3, 4).
The first application of laser as a therapeutic factor dates back to 1960. Laser (LASER) is an abbreviation of 5 words, Light Amplification by the Simulated Emission of Radiation, under the title "amplification of excitation light," which was first proposed by Albert Einstein in 1916 (5).
Laser therapy techniques can help to heal and repair the wound (6). Studies show that laser therapy can help tissue repair by multiplying cells such as fibroblasts, endothelial cells, and keratinocytes (7).
Studies show that in groups treated with laser, a higher amount of collagen is produced, which leads to wound closure. Also, the amount of immune cell secretion was higher in the irradiated group, which helped wound healing (8). In addition to these studies, laser treatment can affect controlling inflammation by reducing edema (9).
Quantitative measurement of inflammatory cytokines at the wound site can help determine the wound's age and the treatment process. Among the inflammatory factors involved in the wound healing process, they mention interleukins, lymphokines, interferons, and Tumor Necrosis Factor-α (9). Studies of increased expression of tumor necrosis factor-α (TNF-α) in the wound site show its role in the proliferation and migration of cells to the wound site (10).
Activated T helper lymphocytes act as a T cell growth factor. Macrophages and keratinocytes at the wound site secrete interleukin 2 (IL-2).
Studies show that IL-2 probably leads to an increase in the activity or number of T lymphocytes, and T lymphocytes also lead to the production of collagen and fibroblast proliferation through the secretion of other cytokines (11).
Vascular endothelial growth factor-A (VEGF-A) is a known factor involved in angiogenesis and is increased by keratinocytes and macrophages at the wound site (12). In addition, it shows that VEGF-A contributes to the wound-healing process by stimulating angiogenesis at the wound site through a paracrine mechanism, and its reduction or degradation causes wound-healing disorders (13). Laboratory studies show that transgenic mice with impaired expression of VEGF-A impair the angiogenesis process of wound healing (14).
2. Objectives
Previous studies showed that wavelengths of 650 nm in the red region and 980 nm in the infrared wavelength have performed well (1, 15). In this study, we have tried to investigate the effect of laser therapy with two intensities of 650 and 980 nm on the adipose tissue fibroblast cell line and, after evaluating cell viability, investigate the changes in cytokines involved in the wound healing process.
3. Methods
The current research is of the laboratory experimental type. This research was carried out in 2022 in the Afra Biology Laboratory under the supervision and support of Islamic Azad University, Dezful Branch.
3.1. Cell Culture
The 3T3-L1 cell line (murine preadipocyte cell line) was obtained from the Pasteur Institute cell bank and cultured in a culture medium containing 90% DMEM and 10% fetal bovine serum. The cells were kept in standard conditions of 37°C, 5% carbon dioxide, and about 80% humidity. In this method, 5 × 105 cells were cultured in a flask, and after the cells filled 70% of the flask, the culture medium was removed and washed twice with PBS, then about 1.5 to 2 mL of DMEM medium Fresh and serum-free was poured on the cells, and the flasks were again transferred to the incubator.
3.2. 3-[4,5-dimethylthiazol-2-yl]-2,5 Diphenyl Tetrazolium Bromide Assay Test
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) test, which is a colorimetric method based on the reduction and breaking of yellow tetrazolium crystals (MTT) by succinate dehydrogenase enzyme and, finally, the formation of insoluble blue crystals. DMSO dissolves these crystals. To perform the test, the flask containing Caca2 cells was removed from the incubator, and after ensuring the health of the cells and the absence of contamination in the culture medium, it was transferred under the hood. Toxicity measurement of the culture medium of 3T3-L1 cells on fibroblast cells that were treated with different laser powers was performed using the MTT method at 0, 24, and 48 hours after the treatment. To prepare MTT stock, 5 mg of this compound was dissolved in 1 mL of phosphate-buffered saline. Cells were cultured in 96-well culture dishes and treated with different drug concentrations and at different times. Four hours before harvesting, 10 µL of MTT was added to the cells. After four hours of keeping in a 37° incubator, the reaction was stopped by adding 0.4 N HCL solution in isopropanol or DMSO. Finally, the absorbance was measured by an ELISA reader at a wavelength of 570 nm.
3.3. Low-power Laser
First, cell suspension prepared from adipose tissue fibroblast cells containing 1 × 104 cells were cultured in 96-well plates. Twenty-four hours after cultivation, the cells were exposed to laser radiation with wavelengths of 650 and 980 nm and distances of 1 cm2 for 10 seconds. The cells were placed in an incubator for 24 hours at a temperature of 37 degrees and an atmosphere of 5% CO2. After 24 hours, 20 microliters of MTT (MELFORD, Etgolistan) were added to one of the wells. They were kept in the incubator for 4 hours, and 100 microliters of DMSO were added to each of the wells to dissolve the resulting formazan. After 30 minutes, the absorbance of formazan was read by an ELISA reader in the absorbance spectrum of 570, and the survival rate was calculated according to the relevant formula.
3.4. Scratch Test
In this study, the effect of the culture medium of 3T3-L1 cells that were subjected to selective power treatment on the healing process of the scratch test was compared to the culture medium of control cells. For this purpose, after the cells reached a density of nearly 90%, the cells were removed in a line in the center of the plate by the sampler head. After washing with PBS solution and removing the separated cells, they were photographed from that part at three different times (0 h, 24 h, and 48 h). The environment without laser radiation was used as a control group. The higher the ability of cells to migrate and invade, the faster they will find the scratched area. The scratch areas were examined by an inverted light microscope (ECLIPS 80i with WG filter, Nikon, Tokyo, Japan) equipped with a digital camera. The percentage of wound healing was calculated based on the reduction of scratch level measured at a specific time as follows:
Wound healing% = time scratch × 100
3.5. Enzyme-linked Immunoassay Assay
Examining the level of cytokines IL-2, TNF-α, and VEGF factor, which plays a key role in angiogenesis and wound healing, was done by the enzyme-linked immunoassay (ELISA) method (kit of Karmania parsgen). These factors were measured in the supernatant culture medium of fibroblast cells treated with the supernatant culture medium of irradiated 3T3-L1 cells compared to the control.
3.6. Data Analysis Methods and Tools
Statistical analysis was performed using Graph Pad Prism 5 statistical software and t-test and ANOVA statistical analysis methods. The significance level of the data was also considered at the 95% level and P values less than 0.05.
4. Results
4.1. Evaluation of Toxicity-induced
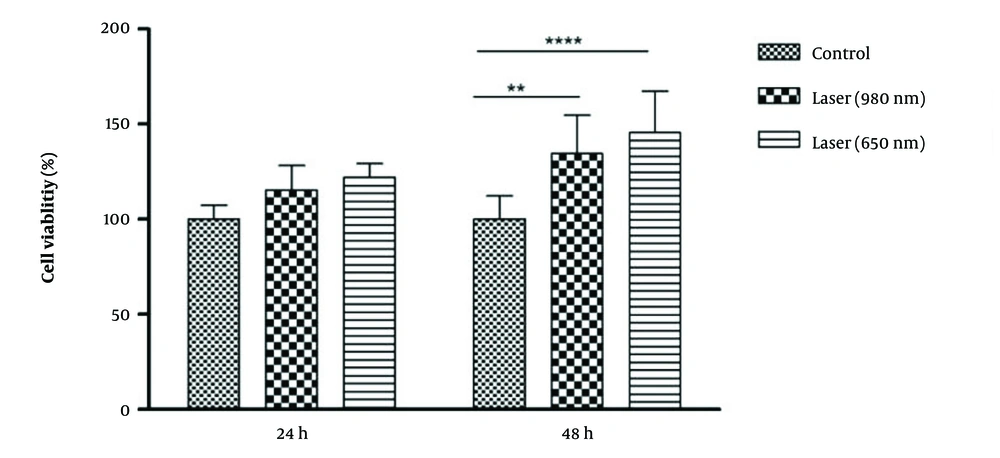
In connection with the comparison of the radiation power of the lasers in 2 time periods, it was found that, in 24 hours, the control group did not have a significant difference with the 980 and 650 laser groups. However, in 48 hours, there was a statistically significant difference between the control group and the 980-laser group (P-Value = 0.01), and laser 650 (P-Value = 0.001) was obtained. The percentage of living cells in the two laser groups was higher than in the control group. The percentage of living cells in the 650-laser group was higher than that of the 980-laser group (Figure 1).
About the time comparison between the groups, the 650-laser group showed a significant difference between the two times of 24 and 48 hours (P-Value = 0.05). In this way, the percentage of living cells after 650 laser irradiation was significantly higher after 48 hours than after 24 hours.
4.2. Evaluation of Interleukin 2 Level
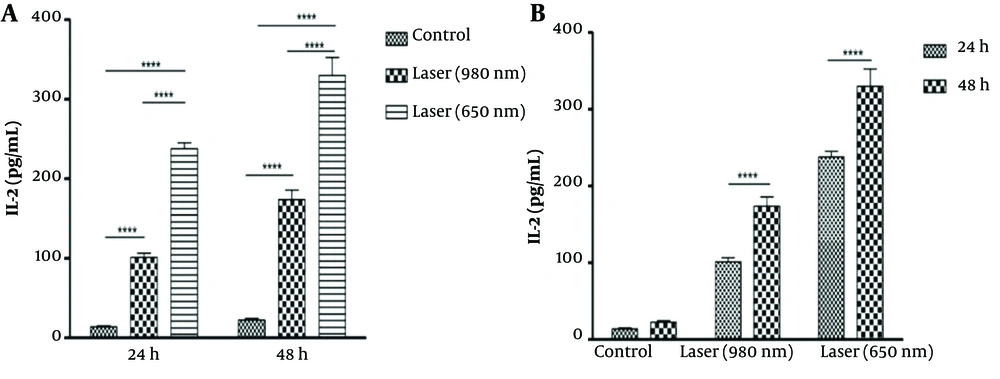
At 24 hours, there was a significant difference in the amount of IL-2 between the control groups and laser 980 (P-Value = 0.001) and laser 650 (P-Value=0.001). Also, there was a significant difference between the two laser groups in that the amount of IL-2 after laser irradiation was 650 more than 980 (P-Value = 0.001) (Figure 2A). At 48 hours, the same results as at 24 hours were significant (P-Value = 0.001). The difference is that the level of IL-2 after laser irradiation was higher at 48 hours than at 24 hours (Figure 2B). The amount of IL-2 after 980 and 650 laser irradiations in 48 hours was significantly higher than 24 hours (P-Value = 0.001). But in general, the amount of IL-2 after laser 650 was higher than laser 980 in both periods.
Comparison of IL-2 levels in 2 time periods of 24 and 48 hours (A). Comparison of the amount of IL-2 after laser irradiation in 2 time periods of 24 and 48 hours (B). The amount of IL-2 after 980 and 650 laser radiation in 48 hours was significantly higher than 24 hours (P-Value<0.001). But in general, the amount of IL-2 after laser 650 was higher than laser 980 in both periods. (****P < 0.0001).
4.3. Evaluation of Tumor Necrosis Factor-α Level
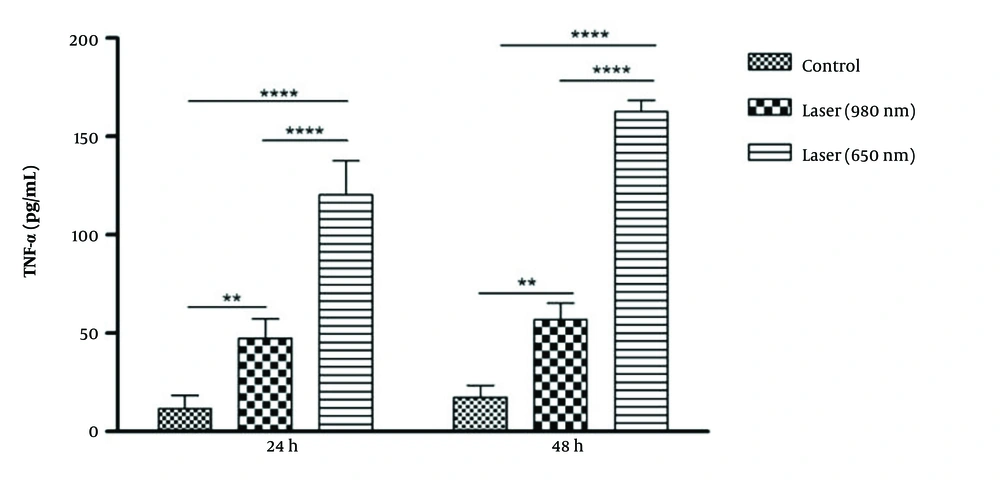
At 24 hours, there was a significant difference in the level of TNF-α between the control groups and laser 980 (P-Value = 0.01) and laser 650 (P-Value = 0.001). Also, there was a significant difference between the two laser groups in that the amount of TNF-α after laser irradiation was 650 more than 980 (P-Value = 0.001) (Figure 3). At 48 hours, the same results as at 24 hours were significant. The difference is that, in general, TNF- levels after laser irradiation were higher in 48 hours than in 24 hours. The amount of TNF-α was significantly higher only after 650 laser irradiations in 48 hours than in 24 hours (P-Value = 0.001).
Comparison of TNF-α level in 2 time periods of 24 and 48 hours. The amount of TNF-α after laser irradiation was higher in 48 hours than in 24 hours. The amount of TNF-α was significantly higher only after 650 laser radiation in 48 hours than in 24 hours (P-Value<0.001). (**P < 0.01; ****P < 0.0001).
4.4. Evaluation of Vascular Endothelial Growth Factor Level
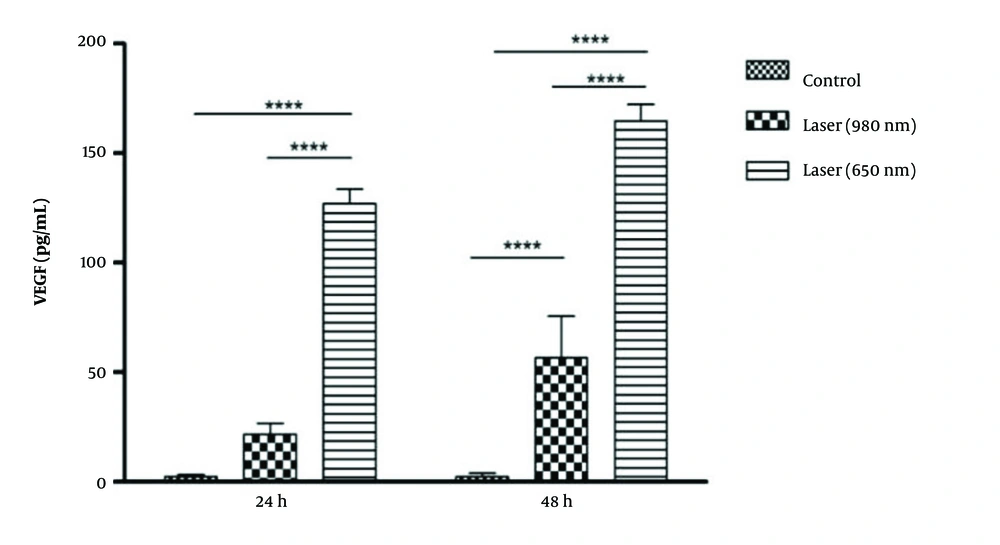
At 24 hours between the 650-laser group, the 980-laser group, and the control group, a significant difference in VEGF was obtained (P-Value = 0.001). So, the amount of VEGF in the 650-laser group was higher than in the 980 and control groups. At 48 hours, the amount of VEGF in the two laser groups was significantly higher than in the control group (P-Value = 0.001). On the other hand, the difference in the amount of VEGF in laser 650 was significantly higher than in laser 980 (P-Value = 0.001) (Figure 4).In general, the amount of VEGF after laser irradiation was 980 (P-Value = 0.01) and 650 (P-Value = 0.001) at 48 hours, significantly higher than at 24 hours.
4.5. Scratch Test
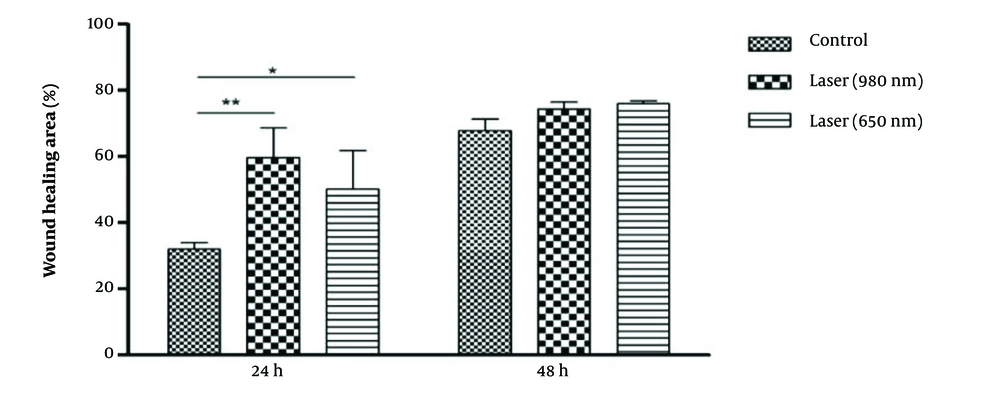
At 24 hours, wound healing with lasers 980 (P-Value = 0.01) and 650 (P-Value = 0.05) was significantly higher than the control group. Also, the percentage of wound healing with 980 lasers was more than 650. No significant results were found after 48 hours (Figure 5). The percentage of wound healing in all three groups of control (P-Value = 0.001), laser 980 (P-Value = 0.05), and 650 (P-Value = 0.01) in 48 hours was significantly higher than 24 hours.
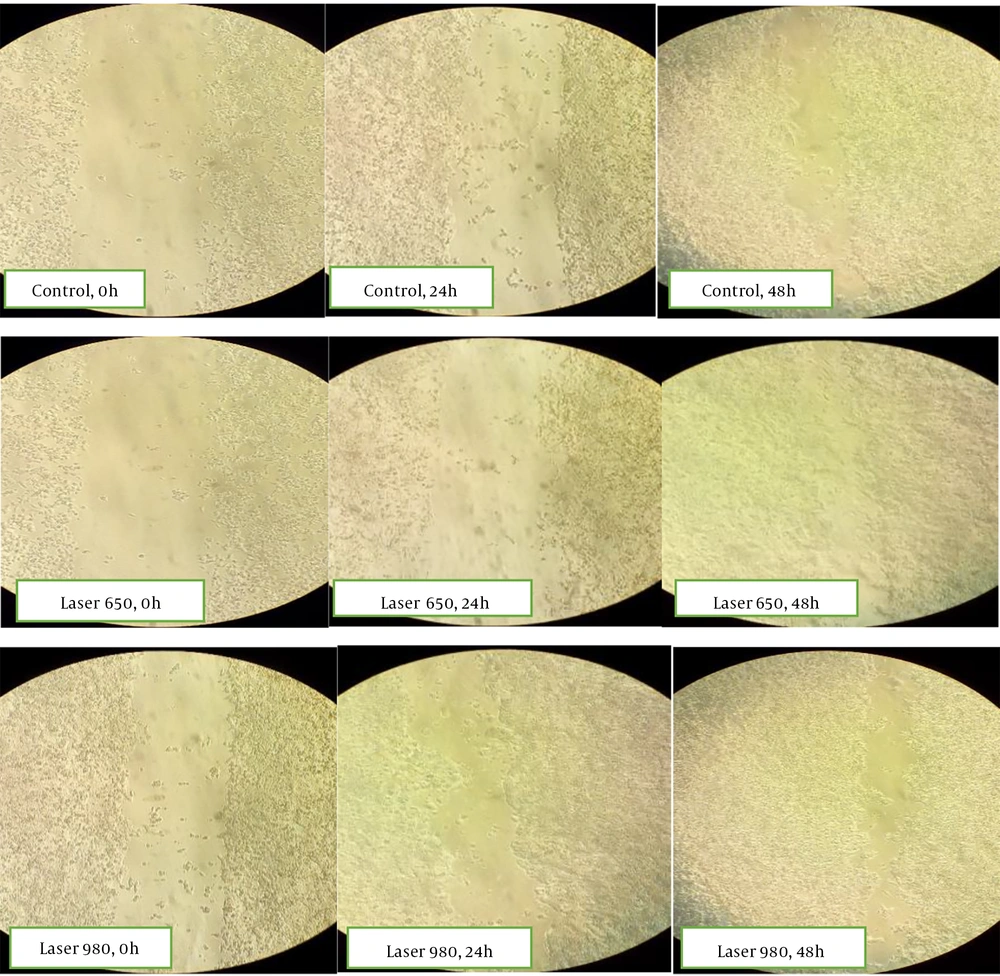
Microscopic images of the wound healing process were recorded at 0, 24, and 48 hours and compared with the laser-treated group. The pictures show that most repair is related to the irradiated cells after 48 hours (Figure 6).
5. Discussion
In this study, we investigated the culture medium isolated from 3T3-L1 cells subjected to low-power laser radiation of 980 and 650 nm on the wound healing process.
The results showed that the percentage of live fibroblast cells after 48 hours, exposed to 650 and 980 lasers, was significantly higher than the control group, not exposed to laser radiation. Also, the percentage of live fibroblast cells with 650 lasers after 48 hours was higher compared to 980 lasers. The wound healing process with laser in 24 hours was more than its healing without laser, and this process was more with laser 980 than 650.
Laser therapy is performed to restore the normal biological function of damaged cells. Considering the pathophysiology of wounds in patients, as well as the positive effects of laser on improving blood flow and tissue repair through stimulation of cell metabolism, the low-power laser has recently been used to recuperate all kinds of wounds (16). Since the mechanisms of low-power laser effect in increasing blood flow and local vasodilatation lead to more reparability of soft tissue cells, and considering that low-power laser does not have significant complications, it is used in a wide range of wounds. This method is used for cases that have suffered burns (17). Studies show the high potential of 650 nm laser therapy. In addition, the power of knowledge is more than growth in this wavelength.
In this study, we have investigated the culture medium isolated from 3T3-L1 cells subjected to low-power laser radiation of 980 and 650 nm on the wound healing process.
The present study showed that the percentage of live fibroblast cells exposed to 650 and 980 lasers was significantly higher than control cells after 48 hours. Also, the rate of live fibroblast cells with 650 lasers after 48 hours was more than 980 lasers. The wound healing process with laser in 24 hours was more than its healing without laser.
Studies prove that light and low-power laser treatment is beneficial for treating wounds because, in addition to the proliferation of epithelial cells, osteoblasts, and fibroblasts, it is also effective in reducing edema in the inflammatory process. Also, light radiation with optimal parameters of wavelength and energy density helps to synthesize collagen and accelerate the process of tissue repair (18).
On the other hand, the percentage of wound healing in all three control, laser 980 and laser 650 group existed more in 48 hours compared to 24 hours. This result can be due to the reparative effects of laser on edema cells and vascularization or due to the antimicrobial effects of laser on the healing process. A proposed mechanism by which laser irradiation stimulates the wound-healing process is the uptake of light energy by mitochondria, which increases cellular energy and stimulates the release of chemical mediators (19).
Generally, after laser radiation, mast cells and macrophages stimulate and release growth factors and cytokines. The proliferation of fibroblasts, endothelial cells, and keratinocytes in unfavorable wound conditions can also shift in this way. Physiological studies in connection with laser therapy have shown that the production and secretion of inflammatory factors have a reciprocal function with neutrophils so that neutrophils secrete protease and elastase enzymes, and being affected by the laser can have a positive effect (20). Also, growth factors released from macrophages mainly control the growth of granulation tissue (21).
Keskiner et al. have shown that photobiomodulation has a positive effect on wound healing by stimulating the production of selective mediators (TGF-b, PDGF, and IL-8) (21). This study is consistent with the results of the present study. In our study, IL-2 and TNF-α increased significantly after 650 laser irradiation for 48 hours compared to 980 laser and the control group.
Contrary to our results, Schlager et al. conducted a study to use a low-power laser to heal burning wounds. They treated the resulting lesions with a continuous wave diode laser with wavelengths of 635, 670, and 690 nm. After pathological examinations, they found no significant difference in the healing process between the groups (22). The reason for the existence of such results, which sometimes contradict each other, can be found in the type of laser used, the elements in lasers, the system of lasers, and also the wavelengths used.
In the present study, after laser treatment, the level of cytokines and VEGF increased, which can indicate the activation of the body's immune pathways to accelerate wound healing. Considering that the VEGF factor also helps the angiogenesis process, the increase in this factor can also indicate the potential of laser therapy to help heal the wound. Furthermore, the results of this study show that laser radiation increases significant changes in chemical mediators that are directly involved in healing, such as increasing the expression of VEGF (a growth factor vital for the formation of granulation tissue).
Considering that these inflammatory mediators increase due to laser radiation, it is concluded that laser, by affecting inflammatory and molecular pathways, potentially plays a significant role in the formation of epithelial cells and wound healing.
5.1. Conclusions
The data obtained from this research show the potential role of laser therapy with intensity of 650 and 980 nm on the wound healing process. The results showed that laser irradiation on adipose tissue fibroblast cell line and wound treatment with cell supernatant solution could potentially help speed up the wound healing process.