1. Background
Cancer is defined as a rapid growth and abnormal division of cells in the body. It is considered as a major health problem and cause of mortality that increases annually (1). Cervical cancer is the third most common cancer in women and associated with disorders in cell cycle control, which normally delays or even stops proliferation (1).
Recently, using new strategies, cervical cancer can be treated by surgery, radiotherapy, chemotherapy, and immunotherapy. However, the side effects caused by them are severe and have a profound impact on the quality of the patients’ lives (2).
Digoxin, a cardiac glycoside, extracted from the leaves of Digitalis lanatafoxglove, has a crucial role in the treatment of congestive heart failure and is widely used. It exerts its therapeutic potential directly and indirectly on the cardiovascular system (3). Recently, it has been proved that some cardiac glycosides such as digoxin have both therapeutic and suppressive potential in cancer therapy (4). Epidemiological data have shown that heart failure patients who take cardiac glycosides have a more benign clinical course with fewer relapses and lower tumor-related mortality compared with those patients who do not receive digoxin (5). Another study declared that the risk of prostate cancer dramatically declined among the patients used digoxin (6).
However, in spite of antitumoral effects of some glycosides based on epidemiological evidences, controversial results about the merits and demerits of application of glycosides in cancer therapy is challenging (7).
It is reported that women who are using digoxin are in danger of breast cancer (8). Of note, no longer lived patients were found among prostate cancer patients taking digoxin in comparison with non-treated men (9). It seems that the glycosides behavior against cancers depends on cancer type, time, and dose of drugs prescription.
The mechanisms by which the glycosides inhibit the cancer cells are unknown. It is suggested that apoptosis could be considered a major consequence of cardiac glycosides on several types of tumor cells (10). Also, it works like many of anticancer pharmacological agents and induces senescence that is considered as a powerful tumor suppressive mechanism (11, 12).
2. Objectives
The present study was conducted to detect cytotoxicity effects of digoxin on HeLa cell line and mechanisms related to its function to help improve our knowledge about its pharmacological and safety issues.
3. Methods
3.1. Cell Line
HeLa cell line was purchased from National Cell Bank of Iran(NCBI) and cultured in RPMI-1640 plus 10% FBS (Fetal bovine serum) in 5% CO2 at 37°C.
3.2. Cell Proliferation
The cells were seeded in 96 well plates with 105 cells /well and treated with 0, 0.1, 0.5, 1, 2, 3, 4, 5, 7, 10, and 15 µM digoxin for 6, 12, 24, and 48 hours. Next, cell proliferation was detected with MTT assay. Briefly, the medium was removed and replaced with 15 µL MTT dye and incubated 3 hours in 5% CO2 at 37°C. Then, DMSO was added to each well, pipetted gently, and read with ELISA reader at 570 nm. Data were presented as OD rate in different groups. The percentage of viability was calculated according to the following formula:
OD of experimental group/OD of control × 100
3.3. Cell Viability
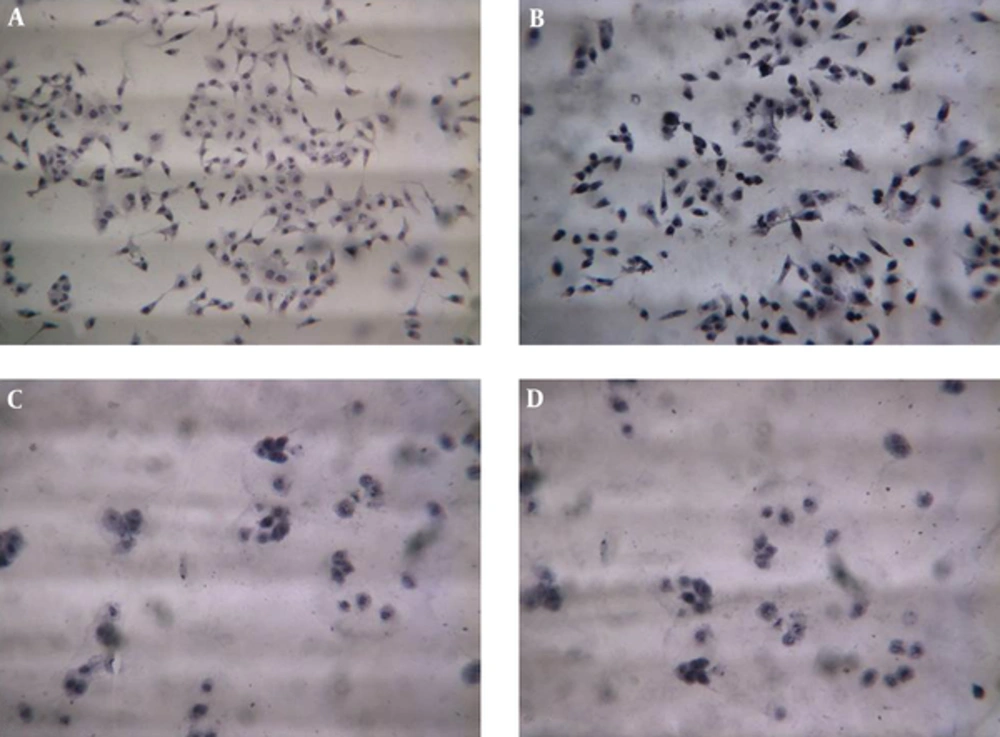
The cells were cultivated in 48 well plates with a density of 105 cells /well and exposed to 0, 0.1, 0.5, 1, 2, 3, 4, 5, 7, 10, and 15 µM digoxin for 6, 12, 24, and 48 hours. The wells were stained with trypan blue, and cell viability was expressed as a percentage (%).
3.4. Colony Assay
To determine the influence of digoxin on cell proliferation, colony assay and cell number in each colony were employed; 103cells/well were seeded in a 48-well plate, and incubated with 4 µM digoxin for 6, 12, 24, and 48 hours at 37°C with 5 % CO2. The medium was changed every 3 days, and colony numbers were reported during 1 week in different groups. Also, the cell number in each colony was counted during the week.
3.5. Hematoxilin Staining
To detect cell nucleus morphology and density, HeLa cells were subjected to hematoxilin staining. The cells were cultivated on slides and incubated with 2, 3, 4, and 10 µM digoxin for 6, 12, 24 and 48 hours, respectively, at 37°C with 5 % CO2. The cells were stained with hematoxilin according to routine protocols. Briefly, the culture medium was replaced with PBS and washed 3 times. Then, the slides were soaked in methanol for 1 hour, followed by washing with water and soaking in hematoxilin for 10 minutes. Finally, the slides were washed with distilled water and the cells were observed under light microscope.
3.6. Gimsa Staining
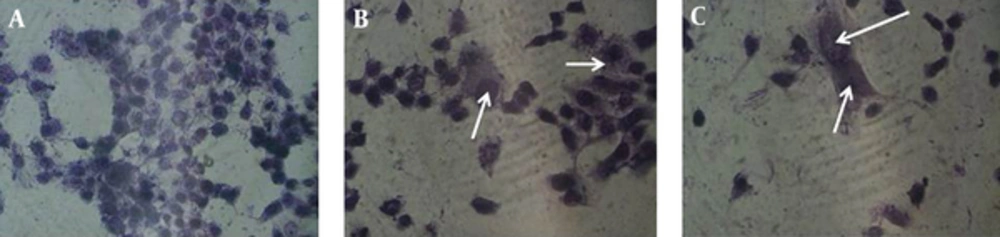
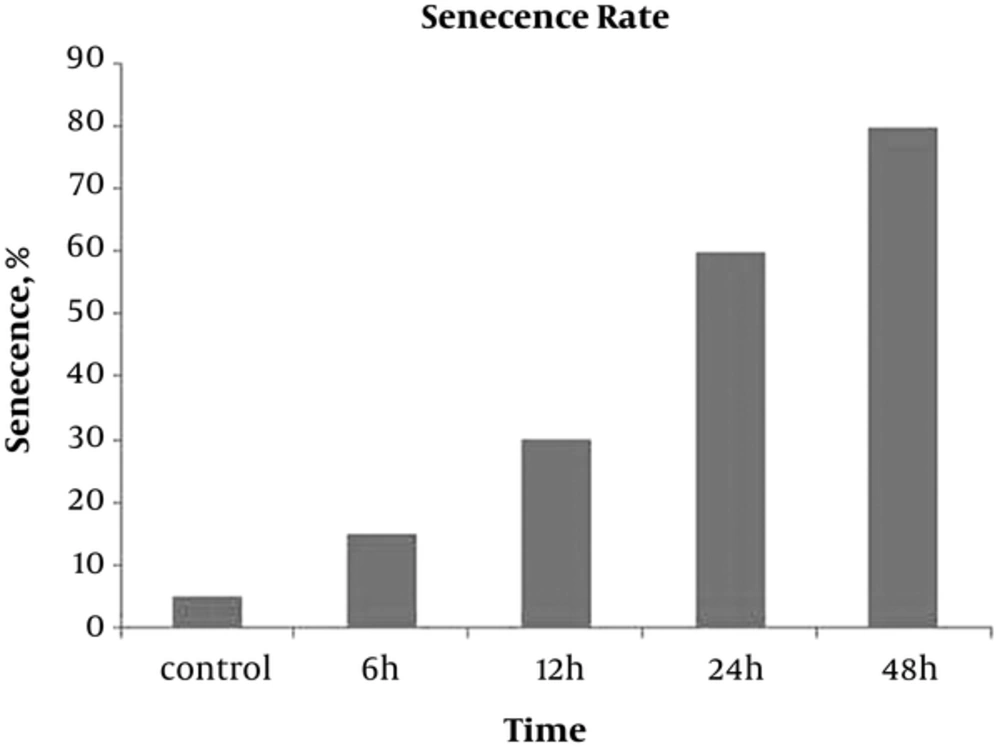
HeLa cells in different control and experimental groups were stained with Gimsa to confirm senescence based on morphology and size of the cells. The cells were cultured in 6 well plates and subjected to digoxin for 6, 12, 24, and 48 hours. Then, the cells were washed with PBS and fixed with methanol. Next, the cells were stained with Gimsa solution for 15 minutes and analyzed under light microscope.
3.7. Statistical Analysis
Data were presented as mean ± standard deviation. One-way ANOVA was used for data analysis using SPSS Version 20 software. The differences between groups were considered statistically significant when the probability level (P value) was less than 0.05 (P < 0.05).
4. Results
4.1. Digoxin Inhibited Proliferation of HeLa Cell
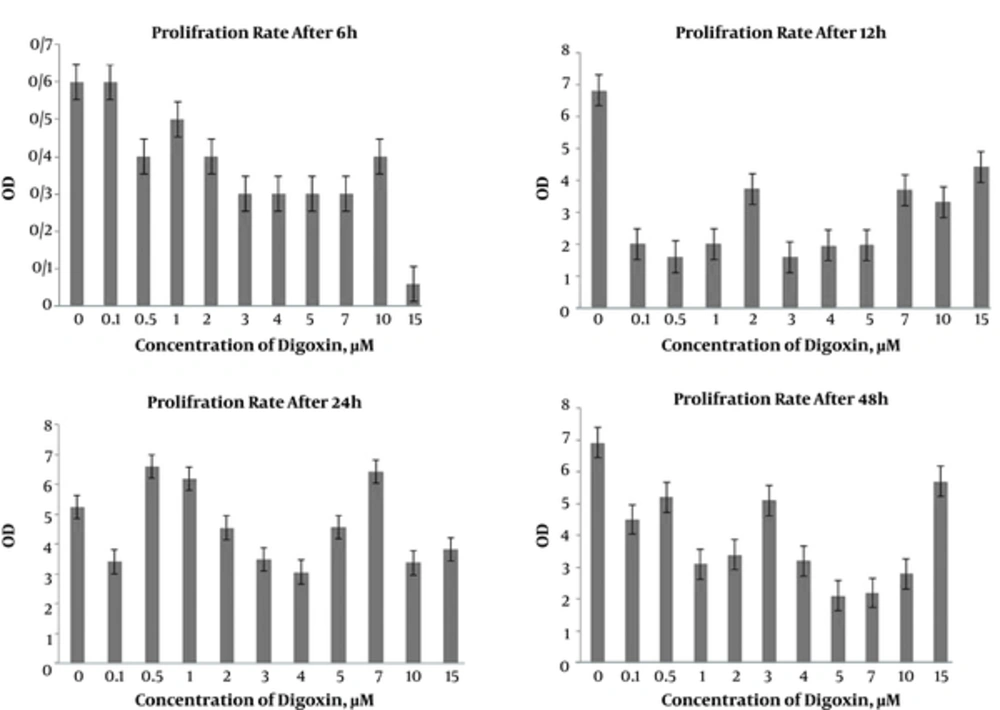
MTT assay was employed to determine the effects of digoxin on proliferation of the HeLa cells. As displayed in Figure 1, there was no significant difference between cell proliferations in control and treated groups with different doses of digoxin after 6 hours. However, the cell proliferations in 2 and 7 µM were significantly lower than the controls after 12 hours (P < 0.05). Furthermore, cell proliferation was significantly inhibited in 4 µM digoxin after 24 hours, compared to the control group (P < 0.05). More time exposure with 0.5, 1, 2, 3, 4, 5, 7, and 10 µM digoxin significantly decreased the cell proliferation after 48 hours (P < 0.05), (Figure 1).
4.2. Digoxin decreased cell viability of HeLa cells.
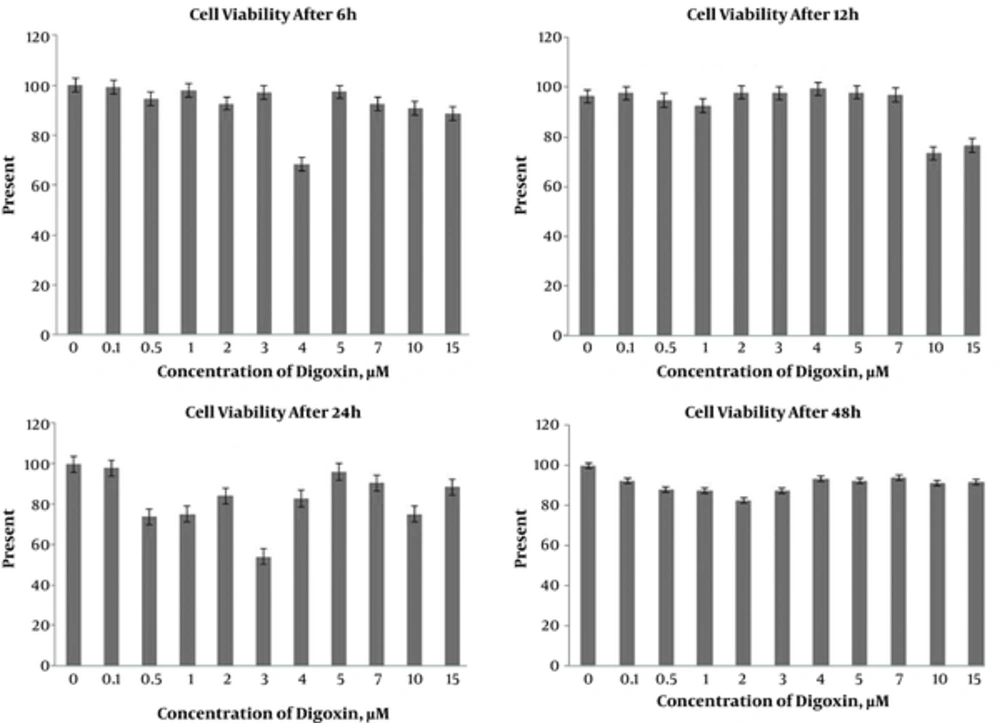
Trypan blue staining was performed to detect the cells viability after exposure to the different concentrations of digoxin. No significant differences were found between control and digoxin treated groups after 6 hours. A significant difference was detected in cell viability of the10 µM digoxin-treated group compared to the controls after 12 hours (P < 0.05). On the other hand, cell viability significantly decreased with 10 µm digoxin treatment (P < 0.05). In addition, after 24 hours, significant differences were found between control and experimental groups with 0.5, 1, and 3 µM digoxin; cell viability was lower in controls in the mentioned doses (P < 0.05). Moreover, the cell viability decreased in all experimental groups compared to the control group after 48 hours (Figures 2 and 3).
4.3. Digoxin Decreased Colony Formation in HeLa Cells
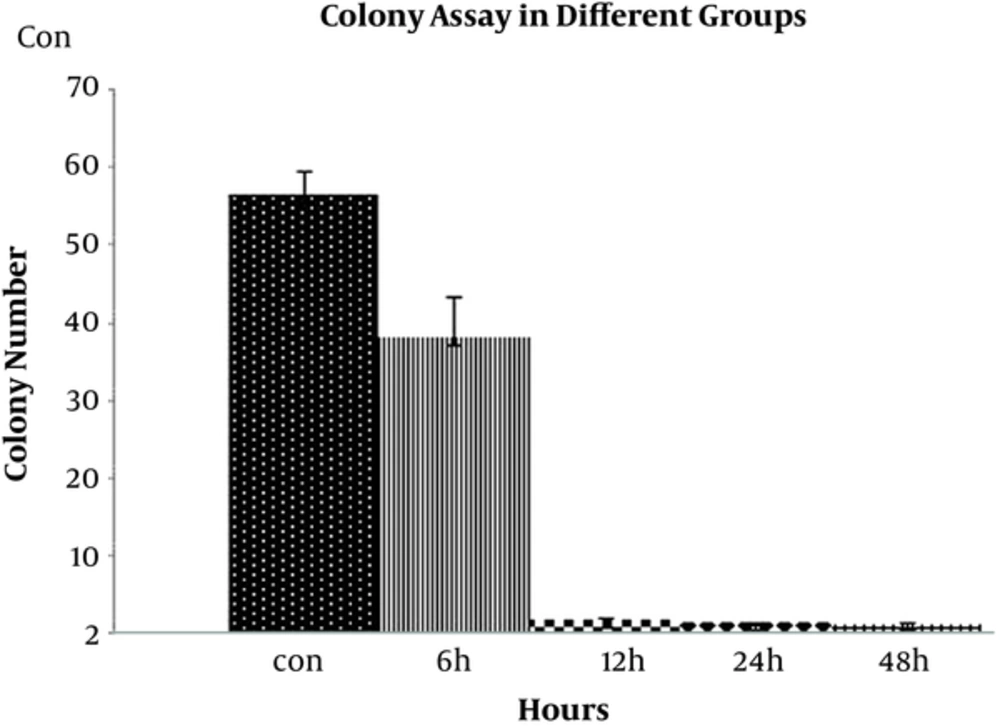
Cultivation of HeLa cell in the presence of 4 µM digoxin significantly decreased colony formation in cells after 6, 12, 24, and 48 hours compared to the control groups. Also, the number of cells in the colonies was significantly decreased in the experimental groups at different times (Figure 4).
4.4. Cell Shape and Nucleus Configuration Changed Pathologically After Digoxin Treatment in HeLa Cell
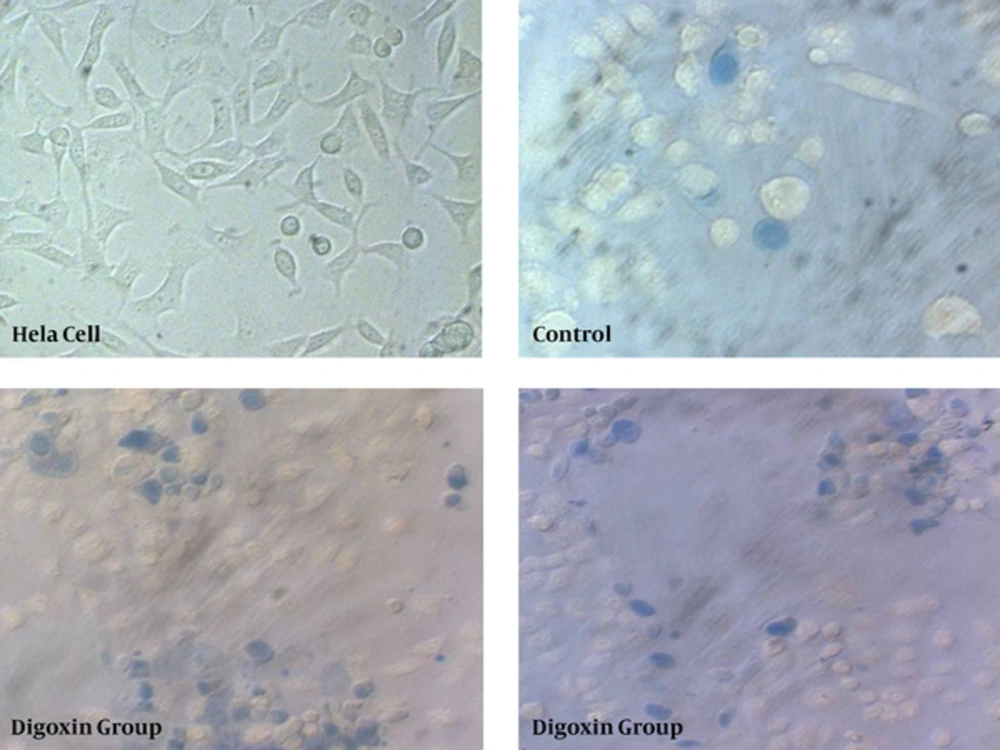
The cells stained with hematoxilin to detect nucleus configuration after treatment with digoxin after 6, 12, 24, and 48 hours. As demonstrated in Figure 5, the cell’s nucleus pathologically changed after 12, 24, and 48 hours of treatment with digoxin. The figure demonstrates that nucleus in the control group was normal and euchromatin. Also, cytoplasm was clear without disruption. However, nucleus in the experimental groups was darker and fragmented and in some cases was enlarged. Cells cytoplasm was disrupted and not visible.
4.5. Digoxin Induced Senescence in HeLa Cells
The gimsa staining was performed to detect giant old cells after exposure to digoxin. Presence of giant multi-nuclear cells indicated senescence induction in digoxin treated cells. Our finding revealed that enlarged cells were observed more in experimental groups after 12, 24, and 48 hours compared with the control group (Figures 6 and 7).
5. Discussion
The current study concentrated on antitumoral effects of digoxin on HeLa cell line and showed that digoxin decreases the proliferation competency of cells. It also suggested that apoptosis and senescence induction might be the contributing mechanisms to digoxin function.
Digoxin, a cardiac glycoside, is used commonly (13). It is an inexpensive and well-tolerated drug used widely as a treatment for heart failure and arrhythmia. Digoxin is still one of the most frequently used medicines in spite of a number of recently developed therapeutic agents for the treatment of heart failure, and remains the first choice of treatment (14).
Reduction of cancer prevalence in patients with cardiac problems who used digoxin recorded in epidemiological studies have suggested the anticancer capability of Na+/K+ ATPase inhibitors in such cancers as prostate, breast, lung, or leukemia (14).
Our findings revealed that digoxin can decrease viability and proliferation of HeLa cell line, meaning that it has antitumoral effects. It is suggested that cardiac glycosides increase the cytosolic Ca2+ concentration and this mechanism explains the antitumor effects of these pharmacological agents in cancer patients (15). In several experimental models, intracellular or extracellular Ca2+ chelators, Ca2+ channel blockers, and calmodulin antagonists are able to delay or decrease apoptosis. Damage of intracellular Ca2+ homeostasis through inhibition of the Na+, and K+- ATPase by cardiac glycosides induce apoptosis in different cell types including tumor cell lines (16).
Some forms of cardiac glycosides in clinically relevant doses could inhibit proliferation and induce apoptosis in prostate cancer cells. These findings suggest that the regulation of intracellular Ca2+ concentration may induce or increase the apoptotic induction in human cancer cells and improve a new target in therapeutic strategies in cancer chemotherapy (17).
Additionally, it is discovered that cardiac glycosides and their derivatives are strong potential antiproliferative agents, tumor-specific, and exert their cytotoxic effects through apoptosis (18, 19).
It has been found that glycosides have the ability to inhibit the activity of Na+, K+-ATPase and lead to intracellular Ca2+ increase (20, 21). Disturbance in regulation of these ions activates a series of intracellular pathways that in turn changes cellular structure or gene expression. A variety of hydrolytic enzymes such as proteases, nucleases, and lipases activate an increase in intracellular Ca2+. This process accounts for effectors of Ca2+ elicited toxicity. Therefore, these changes may have a pivotal role in cellular toxicity (17).
It is believed that gene expression patterns change during cancer development. Hypoxia-inducible Factor 1 (HIF-1) controls oxygen delivery through angiogenesis and metabolic modification to hypoxia via glycolysis in human cancers. Normally, there is hypoxia in tumors, and HIF-1α is overexpressed as a result of intratumoral hypoxia. The findings obtained from biopsies of brain, breast, cervical, esophageal, oropharyngeal and ovarian cancers showed a correlation between HIF-1α overexpression with treatment failure and mortality. In other words, increase in HIF-1 activity accelerates tumor progression and its inhibition could be considered a novel approach in cancer therapy (22, 23).
It has been proved that risk of uterus cancer incidence in women who recently took digoxin significantly increased compared with those patients who did not receive digoxin. However, no change was obtained in cervical cancer risk (24).
Although it has been proved that some glycosides have antitumoral effects, our knowledge is limited, and thus more studies should be conducted to clarify their mechanisms in cell toxicity and molecular field.