1. Background
Obesity is one of the greatest health problems (1). This disorder is an important risk factor for metabolic diseases associated with premature death (2). One of the main causes of overweight and obesity is the consumption of a high-calorie diet and lack of physical activity (3). It is well known that obesity is associated with many diseases related to low-grade chronic inflammation (4-6). Obesity-induced inflammation causes significant disturbances in skeletal muscle structure and function, including impaired muscle metabolism, insulin signaling, atrophy, and reduced hypertrophy (7). Feeding with a high-fat diet (HFD) increases the gene expression of Il-1β and decreases IL-6 mRNA in skeletal muscle (8, 9). A significant increase in the expression of pro-inflammatory cytokines and a decrease in the expression of anti-inflammatory genes in the muscle cause changes in the signaling pathways that disrupt the metabolic functions of the muscle, especially lipid and glucose metabolism (10). For example, a disruption in the skeletal muscle insulin signaling pathway has been reported with a significant increase in the gene expression of pro-inflammatory cytokines in myotubes exposed to palmitic acid (11). Disturbance in the muscle signaling pathway eventually leads to type 2 diabetes (12). It is well known that aerobic exercise is one of the most effective interventions for the prevention and treatment of insulin resistance. Several studies showed that aerobic exercise decreased the gene expression of skeletal muscle pro-inflammatory cytokines, especially IL-1β and TNF-α, under obesity conditions. It increased the gene expression of anti-inflammatory cytokines (13, 14). Since the over-expression of inflammatory mediator genes and the reduction of anti-inflammatory mediators inhibit insulin resistance, aerobic exercises are increasingly considered a protective factor for muscle against insulin resistance (15).
On the other hand, medicinal herbs and supplements can reduce inflammation in obesity due to their bioactive compounds (16-18). One of the plants that has various anti-inflammatory compounds is the pistachio soft hull. It contains anthocyanins, flavan-3-ols, proanthocyanidins, flavonols, isoflavones, flavanones, stilbenes, and phenolic acids (19). The presence of these bioactive compounds gives pistachio soft hull anti-inflammatory properties (20). It has been reported that the compounds in pistachio soft hull inhibit IL-1β gene expression (21-23).
2. Objectives
Aerobic exercise and pistachio soft hull can alter the gene expression of pro-inflammatory and anti-inflammatory mediators in skeletal muscle under conditions of obesity. However, no study has examined the simultaneous effect of these interventions on the expression of IL-6 and IL-1β. The purpose of the current study is to assess the impact of aerobic exercise and pistachio soft hull extract (PSHE) on IL-6 and IL-1β in the soleus muscle of female rats fed a HFD.
3. Methods
3.1. Animals
In an experimental trial, 30 female Wistar rats, weighing between 180 and 200 grams, were purchased from the Pasteur Institute and transferred to the Laboratory Center of Azad Islamic University, Tehran Branch. To allow the rats to adapt to the environment and to control confounding factors, the rats were acclimated for two weeks before the start of the protocols (24, 25). The laboratory environment was prepared according to the following standards: Regulation of relative humidity at 50% ± 10%, regulation of light-dark cycles to 12/12 hours, temperature maintained at 25 ± 2 degrees Celsius, a ventilation system with a silent fan, housing 6 rats in a standard cage, and providing free access to tap water and rat chow. The rats were randomly divided into five groups: 1, normal diet control; 2, HFD control; 3, HFD-aerobic exercise; 4, HFD-PSHE; and 5, HFD-aerobic exercise-PSHE.
3.2. High-Fat Diet Induction
The normal diet of the control group consisted of commercial pellet feed (Behparvar Company, Karaj, Iran) for laboratory mice. To induce a HFD, the normal diet was supplemented with 20% palm oil, 1.5% cholesterol, and 0.25% cholic acid (26).
3.3. Aerobic Exercise Program
The aerobic exercise program consisted of four weeks of five weekly sessions of running on a treadmill. One week before the formal training, the rats were acclimated to the treadmill by running at a speed of 9 m/min for 20 minutes. The intensity and duration of aerobic exercise from the first to the last day were as follows: Moderate-intensity (MET) training sessions at 50 – 60% Vo2max, consisting of five sessions per week with a 5-minute warm-up, a 20-minute exercise period, and a 5-minute cool-down. On the first day of training, the speed started at 16 m/min and, according to the program, increased by 2.5 m/min each week. By the last day, after four weeks, the speed reached 26 m/min (27).
3.4. Pistachio Soft Hull Extract Preparation and Induction
The soft pistachio hull was obtained from Rafsanjan and verified by a botanist. After drying in the shade, it was powdered using a mill. A 100 mg sample was immersed in 5 mL of four solvents (70% acetone, 50% ethanol, 50% methanol, and water) for two hours at room temperature on a shaker, completing the extraction process. The samples were then centrifuged at 3000 g for 10 minutes. The prepared extract in pure liquid form was dissolved in distilled water and administered to the rats via gavage at a dose of 60 mg/kg for four weeks, five times a week (28, 29).
Forty-eight hours after the last interventions (aerobic exercise and PSHE) and after twelve hours of fasting, the rats were anesthetized with a combination of ketamine and xylazine, and the soleus muscle was removed to determine the expression of the IL-6 and IL-1β genes.
3.5. Sacrifice and Collection of Soleus Muscle Tissue
After fasting for 8 – 10 hours, all Wistar rats were sacrificed, and their body weights were measured before tissue harvesting. Once the animals were fully anesthetized and unconscious, in accordance with the Declaration of Helsinki, the rat soleus muscle tissue was quickly removed and washed with PBS (phosphate-buffered saline). Mucus, blood, and excess material were cleaned from the tissues, which were then placed in 2 mL microtubes. The microtubes were placed in a nitrogen tank and stored in an 80-degree freezer until final testing and cell analysis (30).
3.6. Real-time PCR Analysis
The qPCR method was used to study gene expression in soleus muscle tissue. The β-actin reference gene was used as a control, and the expression of other genes was compared to it. First, primers were designed, and then total RNA was extracted from the tissues and converted into cDNA. The cDNA was then amplified using PCR, and the expression of these genes was analyzed. RNA extraction was carried out manually using Trizol reagent prepared by the Kiazist Company, following the currently accepted standard procedure for the trizol method. cDNA synthesis was performed using Oligo dT primers and according to the instructions of the German Fermentase kit. The primers were designed using Gene Runner version 6.5 (Table 1) (31).
| Variables | Reverse (5’ -> 3’) | Forward (5’ -> 3’) |
|---|---|---|
| IL-1β | CACGGTTTTCTTATGGCTCTG | CTATTCCTAATGCCTTCCCCAG |
| IL-6 | TGTCCATCAGCCTCCAATTC | ACCAAGACCATCCAACTCATC |
| β-actin | GCTTAGGCATAGCACACTAGG C | ACCGTGAAAAGATGACCCAG |
Abbreviations: L-1β, interleukin 1 beta; IL-6, interleukin 6; β-actin, Beta-actin.
3.7. Statistical Method
The Shapiro-Wilk test was used to determine the normality of the data distribution. After confirming the data were normally distributed, an independent t-test was used to test the differences between the healthy control group and the HFD group. A two-way analysis of variance was used to examine the differences between the other groups. Bonferroni's post hoc test was also used to determine the main interaction effect of aerobic exercise and PSHE on IL-1β and IL-6. A significance level of P < 0.05 was considered for all calculations. All analyses were performed with SPSS version 25.
4. Results
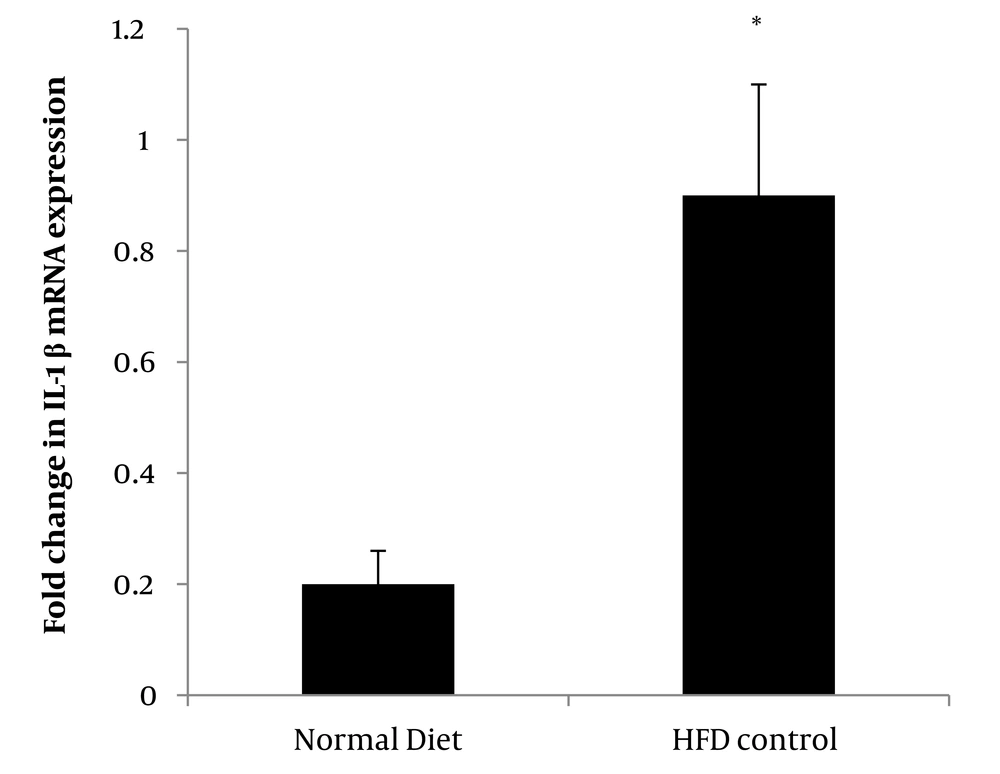
Induction of HFD significantly increased IL-1β gene expression (P = 0.001) in the control group compared to the normal diet control group (Figure 1).
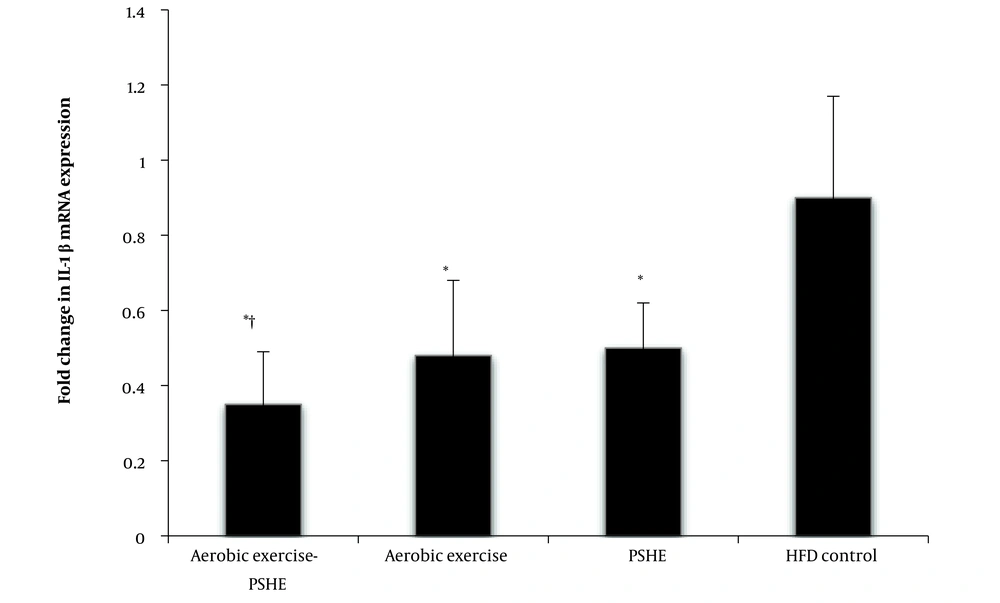
IL-1β gene expression in the aerobic exercise (P = 0.001), PSHE (P = 0.003), and aerobic exercise-PSHE (P = 0.001) groups was significantly lower than in the HFD control group. The interaction of aerobic exercise and PSHE on the expression of the IL-1β gene in the soleus muscle of rats fed an HFD was significant (P = 0.001), indicating that these two interventions enhance each other's effect in reducing the expression of this gene (Figure 2).
Effect of aerobic exercise and PSHE on IL-1β gene expression of soleus muscle in female Wistar rats. PSHE, pistachio soft hull extract; HFD, high-fat diet. Data represent mean ± SD (n = 6). Two-way ANOVA, followed by the post hoc test of Bonferroni. * Denotes significant differences to the HFD control group, and † denotes significant interaction of aerobic exercise and PSHE.
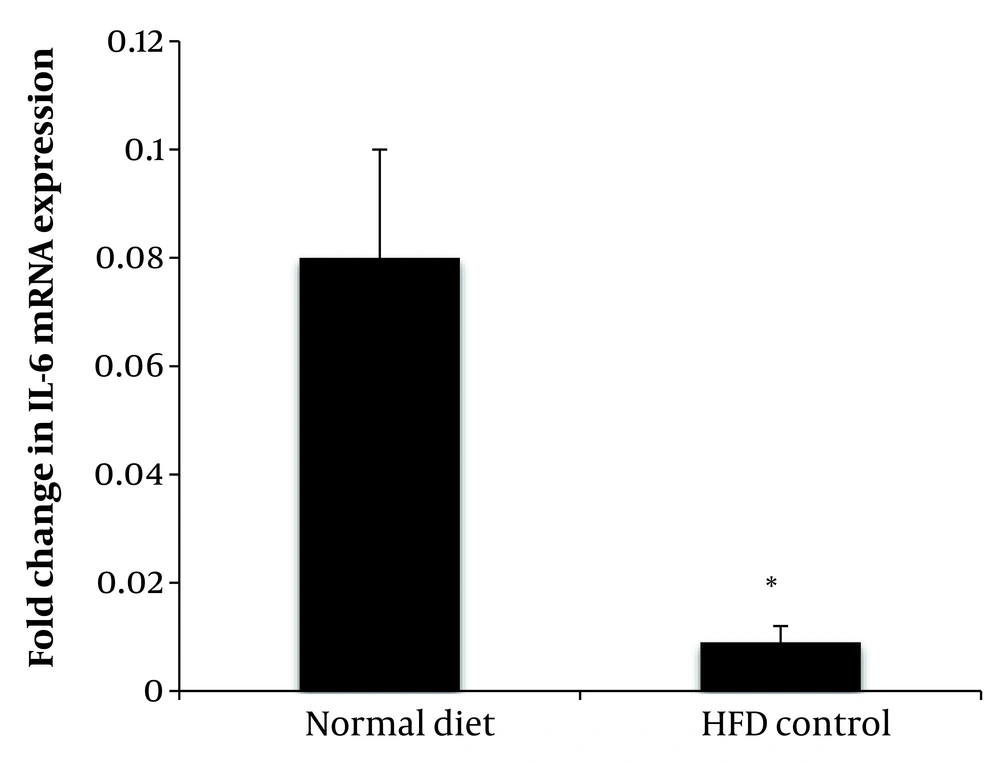
The expression of the Il-6 gene of soleus muscle in the group fed with HFD was significantly lower than the group fed with a normal diet (P = 0.001) (Figure 3).
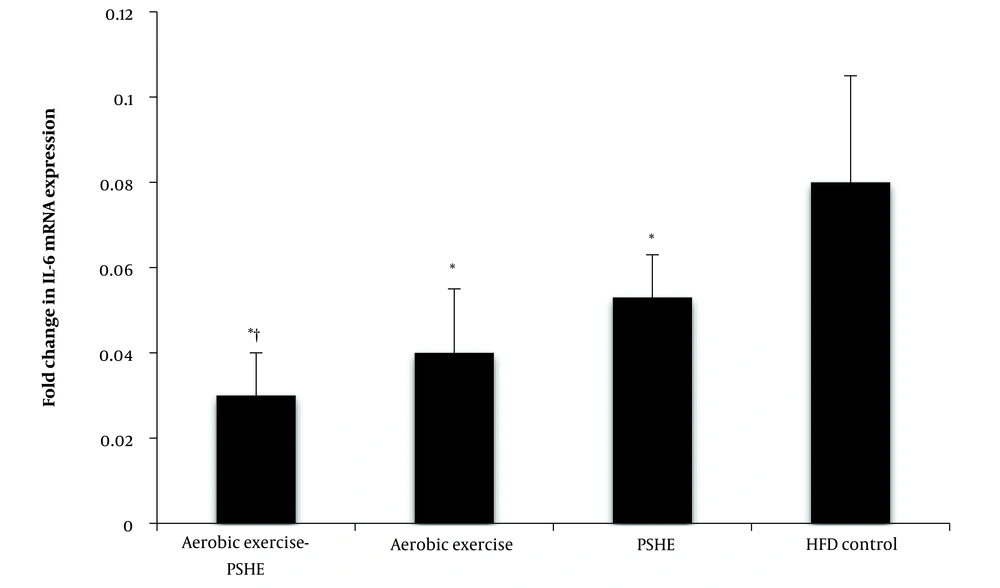
Aerobic exercise (P = 0.002), PSHE (P = 0.002), and the combination of aerobic exercise- PSHE (P = 0.003) caused a significant increase in IL-6 gene expression compared to the HFD control group. The interaction of aerobic exercise and PSHE was significant in the increase of IL-6 gene expression (P = 0.003) (Figure 4).
Effect of aerobic exercise and PSHE on IL-6 gene expression of soleus muscle in female Wistar rats. PSHE, pistachio soft hull extract; HFD, High-fat diet. Data represent mean ± SD (n= 6). Two-way ANOVA, followed by the post hoc test of Bonferroni. * Denotes significant differences to the HFD control group, and † denotes significant interaction of aerobic exercise and PSHE.
5. Discussion
The first finding of this study showed that feeding with a HFD increased IL-1β and decreased IL-6 gene expression in the soleus muscle. Eating a HFD causes low-grade chronic inflammation and insulin resistance (32). From a molecular perspective, obesity increases tissue and systemic inflammation due to increased gene expression of pro-inflammatory mediators at the cellular level (33-35). Skeletal muscle, like other tissues, is affected by a HFD. Increased inflammation, oxidative stress, and disturbance in skeletal muscle metabolism with HFD have been reported in rodents (36, 37). Like other tissues such as the liver, brain, and adipose tissue, feeding with a HFD is generally associated with increased gene and protein expression of inflammatory mediators such as IL-1β and TNF-α in skeletal muscle.
The exact molecular mechanism of increased IL-1β expression in skeletal muscle following HFD is not well known, but possible mechanisms have been proposed. One suggested mechanism involves the activation of the cytoplasmic nucleotide-binding oligomerization domain-like receptor family (NOD-like) pyrin domain containing 3 (NLRP3) inflammasome (9). Pyrin domain containing 3 is associated with increased inflammation and insulin resistance. Fatty acids in a HFD, especially palmitic acid, activate the TLR4/NFkB signaling pathway by acting on the TLR4 receptor (38). The activation of NFkB leads to the activation of NLRP3, which then triggers the expression of the IL-1β gene (9). Additionally, feeding with a HFD activates caspase-1 and increases IL-1β gene and protein expression (39).
In the present study, aerobic exercise decreased IL-1β gene expression. This exercise increases the uptake and oxidation of plasma fatty acids by active muscles, thus reducing the activation of the TLR4/NFkB pathway and subsequently decreasing IL-1β mRNA expression. Increased oxidative stress is one of the factors that activate inflammatory mediators in skeletal muscle tissue (8). It seems that in the present study, aerobic exercise inhibited IL-1β gene expression by reducing oxidative stress.
On the other hand, PSHE also decreased IL-1β gene expression. Pistachio soft hull extract bioactive compounds have hypolipidemic properties. It is reported that anthocyanins (40), proanthocyanidins (41), and flavonols (42) exert their hypolipidemic effects by inhibiting the absorption of fats in the small intestine and reducing circulating lipids. As a result, these compounds can decrease the expression of IL-1β mRNA by reducing circulating fatty acids and subsequently reducing the activation of the TLR4/NFkB pathway. Therefore, it seems that PSHE reduces IL-1β gene expression by decreasing circulating lipids and affecting signaling pathways. Additionally, PSHE bioactive compounds, due to their high antioxidant properties, reduce the oxidative stress caused by HFD and subsequently lower the expression of IL-1β.
The significant finding of this study was the notable interaction of aerobic exercise and PSHE on the reduction of IL-1β gene expression. It appears that aerobic exercise and PSHE each reduced fatty acids and oxidative stress through different mechanisms, thereby decreasing IL-1β gene expression in the soleus muscle.
Another finding of this study showed that HFD decreased IL-6 gene expression in the soleus muscle. IL-6 is an inflammatory cytokine expressed as an adipokine in adipose tissue and released into the circulation, which is associated with tissue damage and the development of many metabolic diseases (43). Since IL-6 is also expressed in skeletal muscle tissue and responds to muscle contractions, it has attracted the attention of researchers as a myokine with anti-inflammatory properties. During muscle contractions, IL-6 expression increases, enters the blood circulation, and enhances lipolysis, uptake, and oxidation of fatty acids by skeletal muscles (44). It has been reported that skeletal muscle IL-6 expression decreases due to a HFD. Performing aerobic exercises can counteract the inhibitory effect of a HFD on IL-6 gene expression and increase IL-6 expression in skeletal muscle (7). In the present study, aerobic exercise increased IL-6 gene expression. It seems that aerobic exercise improves the myokine profile by increasing the uptake and oxidation of fatty acids and stimulating IL-6 gene expression. Muscle contractions caused by aerobic exercises increase the level of cytosolic calcium ions and enhance the expression of IL-6 through the activation of MAPK and calcineurin (45).
In the present study, PSHE also increased the expression of the IL-6 gene in the soleus muscle. The reduction of plasma fatty acids caused by the compounds in PSHE led to a change in the activation pattern of myokine gene expression, especially IL-6. Both aerobic exercise and PSHE were able to amplify each other's effects on skeletal muscle IL-6 gene expression through different molecular mechanisms, indicating the synergistic effect of these two interventions on the expression of this gene.
5.1. Conclusions
A HFD has been implicated in promoting inflammation within skeletal muscle, primarily by elevating the expression of pro-inflammatory genes such as IL-1β and IL-6. Aerobic exercise, characterized by sustained and rhythmic physical activity, has demonstrated efficacy in counteracting the deleterious effects of HFD-induced inflammation. It effectively downregulates the expression of IL-6 and IL-1β genes in skeletal muscle tissue. Similarly, PSHE has emerged as a noteworthy agent in attenuating skeletal muscle inflammation. Furthermore, combining aerobic exercise with PSHE represents an innovative approach, synergistically amplifying their individual benefits.