1. Background
Gastric cancer ranks as the fourth leading cause of cancer-related deaths globally and is among the most common malignancies. The development and progression of gastric cancer are influenced by both genetic and environmental factors (1). Despite intensive efforts to improve mortality rates, patients with advanced or metastatic gastric cancer continue to face poor prognoses due to the limited effectiveness of widely used cytotoxic chemotherapy (2, 3). Therefore, understanding the molecular mechanisms driving gastric cancer progression is crucial for developing novel targeted therapies.
Several studies indicate that cancer stem cells (CSCs) are key players in cancer initiation and progression (4). Cancer stem cells (CSCs) possess both self-renewal and pluripotency capabilities. They are believed to originate from deregulated stem cells or dedifferentiated progenitor cells, as evidenced by the presence of stem cell factors such as NANOG, OCT-4, and SOX2 in both stem cells and CSCs. Recent findings show that these core transcription factors are vital not only in embryonic stem cells (ESCs) but also in reprogramming somatic cells into ESC-like states, as demonstrated by induced pluripotent stem cells (iPSCs) (5-7).
It has been shown that CSCs exhibit a higher degree of differentiation compared to non-stem cells, leading to the acquisition of stem cell-like properties following transplantation. This is primarily attributed to the process of epithelial-to-mesenchymal transition (EMT). Alternatively, it is hypothesized that CSCs may arise from non-malignant cells through morphological changes induced by somatic oncogene mutations (8, 9).
Various subpopulations of cancer cells have been reported to contain surface stemness markers, such as CD44, CD133, and ALDH1. These markers play distinct roles in tumor progression, metastasis, and resistance to radiotherapy. For instance, tumorigenesis in Head and Neck Squamous Cell Carcinoma (HNSCC) is linked with the expression levels of CD44 and NANOG (10, 11). At present, CD133, CD44, and CD24 are among the most frequently studied and are considered representative markers of cancer stem cells (12).
The ability of stem cells to self-renew and differentiate is intrinsically associated with cell-cycle progression, which is vital for tissue specification, organ homeostasis, and potentially tumorigenesis (13). For example, previous studies have shown that the human stem cell marker NANOG can regulate cell proliferation through G0/G1 cell cycle arrest. NANOG downregulation led to cell cycle arrest via increased expression of p27 and P21, resulting in reduced proliferation and differentiation (14).
Hox Transcript Antisense Intergenic RNA (HOTAIR), a long non-coding RNA (lncRNA), affects various aspects of cancer pathways, including proliferation, migration, and drug resistance (15). A significant correlation has been observed between HOTAIR expression in tumor tissues and factors such as lymph node metastasis, TNM stage, and invasion in gastric cancer (16, 17). Furthermore, high HOTAIR expression is associated with a poor survival rate in gastric cancer patients (18). Both in vitro and in vivo studies have shown that HOTAIR can enhance cancer stem cell activity. For instance, HOTAIR influences CSCs through the regulation of SOX2 expression in breast cancer and affects processes such as self-renewal capacity. Notably, HOTAIR tightly regulates the transcription of p53 (19, 20). However, the impact of HOTAIR on stemness and cell cycle markers in gastric cancer remains unclear. Understanding HOTAIR's role in inducing stemness and affecting cell progression in gastric cancer cells may provide significant insights for patient treatment.
2. Objectives
The primary objective of this study is to investigate the correlation between alterations in HOTAIR expression and the presence of stem cell features in cancer cells. Additionally, the study aims to examine the relationship between changes in the expression of stem cell marker genes, which are crucial in manifesting stem cell characteristics in cancer cells. This research focuses on the effect of HOTAIR on key stemness markers in AGS and MKN45 gastric cancer cell lines, which were transfected with siRNA and overexpression vectors of HOTAIR. Flow cytometry was utilized to assess HOTAIR's role in modulating the cell-surface expression of CD44 and CD24 and inducing cancer stem cell-like properties in gastric cancer. The study was followed by expression analysis of two notable markers, NANOG and P21, as indicators of stemness.
3. Methods
3.1. Cell Lines and Culture Conditions
Human gastric cancer (GC) cell lines MKN45 and AGS were obtained from the Pasteur Institute of Iran (Tehran, Iran). The cells were cultured in Gibco RPMI 1640 medium (Gibco, USA) supplemented with 10% FBS (Invitrogen, USA) and 1% penicillin and streptomycin (Invitrogen). We incubated the cells at 37°C with 5% CO2 in a humidified atmosphere. Subculturing was performed upon reaching confluence. An inverted microscope was utilized to monitor cell growth.
3.2. Knockdown of HOTAIR in AGS and MKN45 Cell Lines
In this study, siRNA-HOTAIR (Hs02_00380445) was obtained from Sigma. AGS and MKN45 cells were transfected with 50 nM of si-HOTAIR and siNC (MISSION® siRNA Universal Negative Control #1, SIGMA/SIC001) as the control. According to the manufacturer's protocol, AGS and MKN45 cells were seeded in 24-well plates. After 24 hours and reaching 50% confluence, the cells were transfected with Lipofectamine® 2000 (Invitrogen). The cells were harvested 48 hours post-transfection. The efficiency of siRNA transfection was evaluated using real-time PCR. A Mock control, consisting of the transfection mix without siRNA, was used for comparison.
3.3. Upregulation of HOTAIR in AGS and MKN45 Cell Lines
The overexpression vector (pBabe-HOTAIR) and control vector (pBabe-vector) were provided by the Division of Cancer Stem Cell, Miyagi Cancer Center, Japan (21). AGS and MKN45 cells were separately transfected with pBabe-HOTAIR and pBabe-puro vectors. Cells were seeded in 24-well plates and, after 24 hours at 50% confluence, transfected with Lipofectamine® 2000 (Invitrogen). The cells were harvested 48 hours post-transfection. The efficiency of overexpression vector transfection was verified using real-time PCR. These experiments were conducted in triplicate, using control vectors as negative controls. The Mock control included the prepared transfection mix without any vector.
3.4. Flow Cytometry
Flow cytometry analysis of AGS and MKN45 cells was conducted after digestion with 0.25% trypsin, followed by three washes with PBS. The cells were then resuspended in 100 µL of PBS and stained with anti-CD44-FITC and anti-CD24-PE at room temperature for 40 minutes. After three additional PBS washes, the samples were resuspended in 200 µL of PBS. PE-anti-CD24 and FITC-anti-CD44 alone were utilized as isotype controls for the control cells. A FACS Aria III flow cytometer (BD Biosciences, San Jose, CA, USA) was employed to determine the percentages of CD44+/CD24+, CD44+/CD24-, CD44-/CD24+, and CD44-/CD24- cells.
3.5. RNA Extraction and cDNA Synthesis
Total RNA was extracted using RNX (CinnaGen, Iran) following the manufacturer's instructions. After extraction, RNA was treated with DNaseI (Sigma) and stored at -80°C. Reverse transcription of RNA into cDNA was then performed using a cDNA Synthesis Kit (Takara, Japan), according to the manufacturer's instructions.
3.6. Real-time PCR
Primers were designed using Gene Runner software (version 6.0; ) and Primer-Blast based on cDNA sequences obtained from the Gene Bank. The primer sequences used in this study are listed in Table 1.
| Target Gene | Primer Name | Sequence |
|---|---|---|
| HOTAIR | F | 5'-AGGCCCTGCCTTCTGCCT-3' |
| HOTAIR | R | 5'-TGCTCTCTTACCCCCACGGA-3' |
| HPRT1 | F | 5'-GGACTTTGCTTTCCTTGGTCAG-3' |
| HPRT1 | R | 5'-GTCAAGGGCATATCCTACAACA-3' |
| NANOG | F | 5'-CCT ATG CCT GTG ATT TGT GG-3' |
| NANOG | R | 5'-AGT GGG TTG TTT GCC TTT G-3' |
| P21 | F | 5'-TGGAGACTCTCAGGGTCGAAA-3' |
| P21 | R | 5'-CGGCGTTTGGAGTGGTAGAA-3' |
Primers Used in This Study
Real-time PCR was carried out using SYBR-Green PCR Mix (Takara) on an ABI Step-One system (Applied Biosystems, USA). The total reaction volume was 10 µL, comprising 1 µL of cDNA, 0.5 µL of forward primer (10 µM), 0.5 µL of reverse primer (10 µM), 5 µL of 2x SYBR Green PCR master mix, and 3 µL of RNase-free water. The PCR conditions included a 95°C denaturation for 5 minutes, followed by 40 cycles of 5 seconds at 95°C and 30 seconds of annealing/extension at 60°C. The HPRT1 gene was used as the internal control.
3.7. Statistical Analysis
The GraphPad Prism 9 software was utilized for statistical analysis. Data are presented as mean ± standard deviation. One-way ANOVA was employed for the analysis of the data. A P-value of less than 0.05 was considered to indicate statistical significance. All experiments were conducted in triplicate for each sample.
4. Results
4.1. Downregulation and Upregulation of HOTAIR
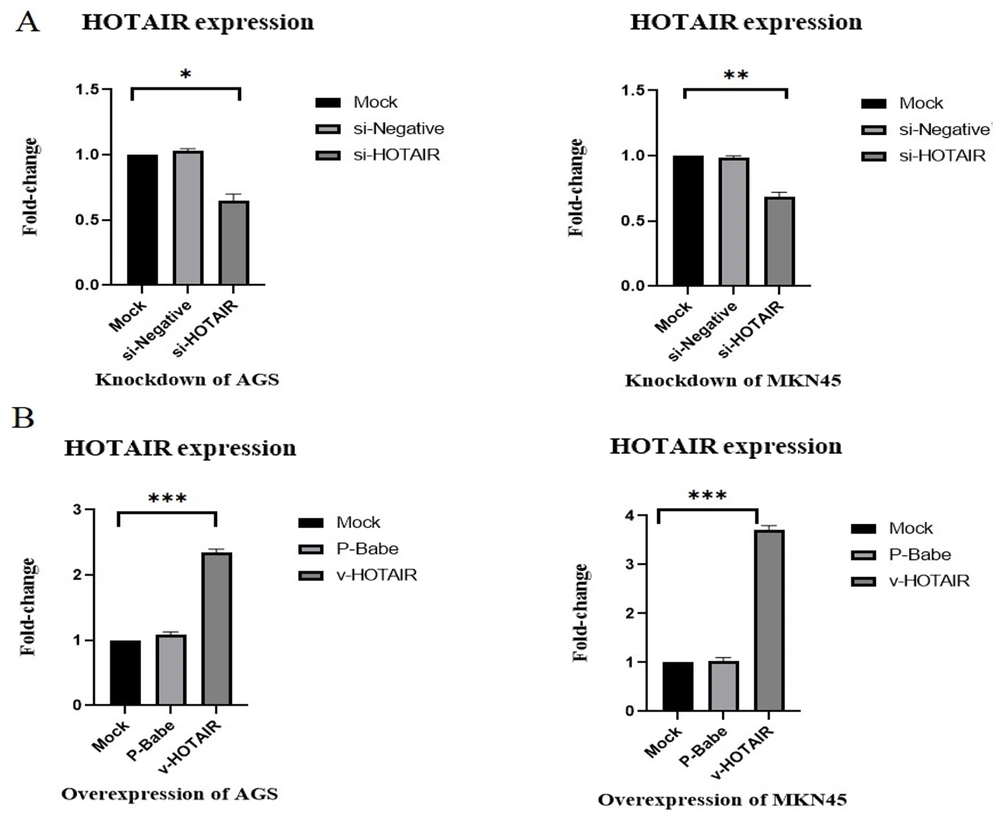
To explore the role of HOTAIR in gastric cancer cells, HOTAIR siRNA (siHOTAIR) was transfected into AGS and MKN45 cell lines using Lipofectamine 2000. Transfection of siHOTAIR into AGS and MKN45 cells resulted in a significant knockdown of HOTAIR expression, with reductions of 32% in AGS and 27% in MKN45 cells (P < 0.05; P < 0.01; Figure 1). In contrast, cells transfected with NC siRNA exhibited no significant difference in HOTAIR expression levels compared to the mock cells.
HOTAIR expression levels in gastric cancer cell lines analyzed by qRT-PCR. A, The expression of HOTAIR in AGS knockdown cells was reduced by an average of 32%, and in MKN45 knockdown cells, it was reduced by an average of 27% compared to mock cells. B, AGS cells with overexpressed HOTAIR showed approximately 2.35-fold higher expression, and MKN45 cells with overexpressed HOTAIR exhibited about 3.7-fold higher levels than mock cells (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).
Additionally, to assess the role of upregulated HOTAIR in the development of gastric cancer, we generated gastric cancer cells with overexpressed HOTAIR. Quantitative Reverse Transcription PCR (qRT-PCR) analysis revealed that HOTAIR levels were significantly elevated in AGS and MKN45 cells transfected with the HOTAIR overexpression vector compared to the mock cells, with increases of up to 2.35-fold in AGS and up to 3.7-fold in MKN45 cells (P < 0.001; Figure 1). Cells transfected with the pBabe-vector showed no significant change in HOTAIR expression levels compared to the mock cells.
4.2. Flow Cytometry Analysis Revealed that HOTAIR Impacts CD44 and CD24 Stemness Markers in Gastric Cancer Cells
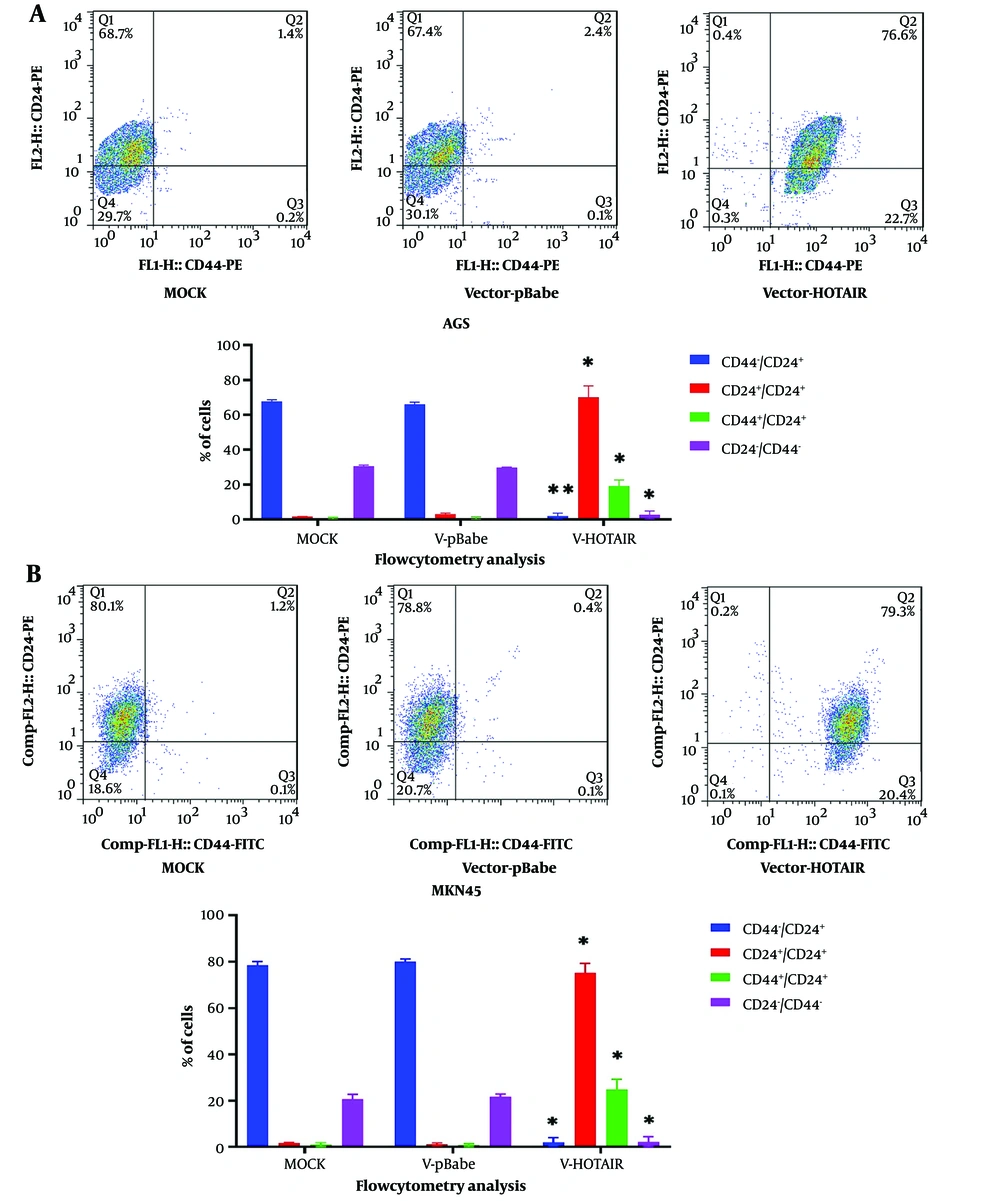
The CD44/CD24 markers of AGS and MKN45 cells were evaluated using flow cytometry. The aim of our study was to investigate the influence of HOTAIR on stemness in cancer cells, which was conducted by measuring the expression levels of CD24 and CD44 on the surface of AGS and MKN45 cells. As depicted in Figure 2, there was an increase in CD44+CD24+ cells after 48 hours of HOTAIR overexpression.
Impact of HOTAIR upregulation on CD44 and CD24 markers. dot plots and percentages of CD44+/CD24+, CD44+/CD24−, CD44−/CD24+, and CD44−/CD24− subpopulations in A, AGS; and B, MKN45 cell lines, as determined by flow cytometry. Each experiment was conducted in triplicate for each sample (*P < 0.05 and **P ≤ 0.01).
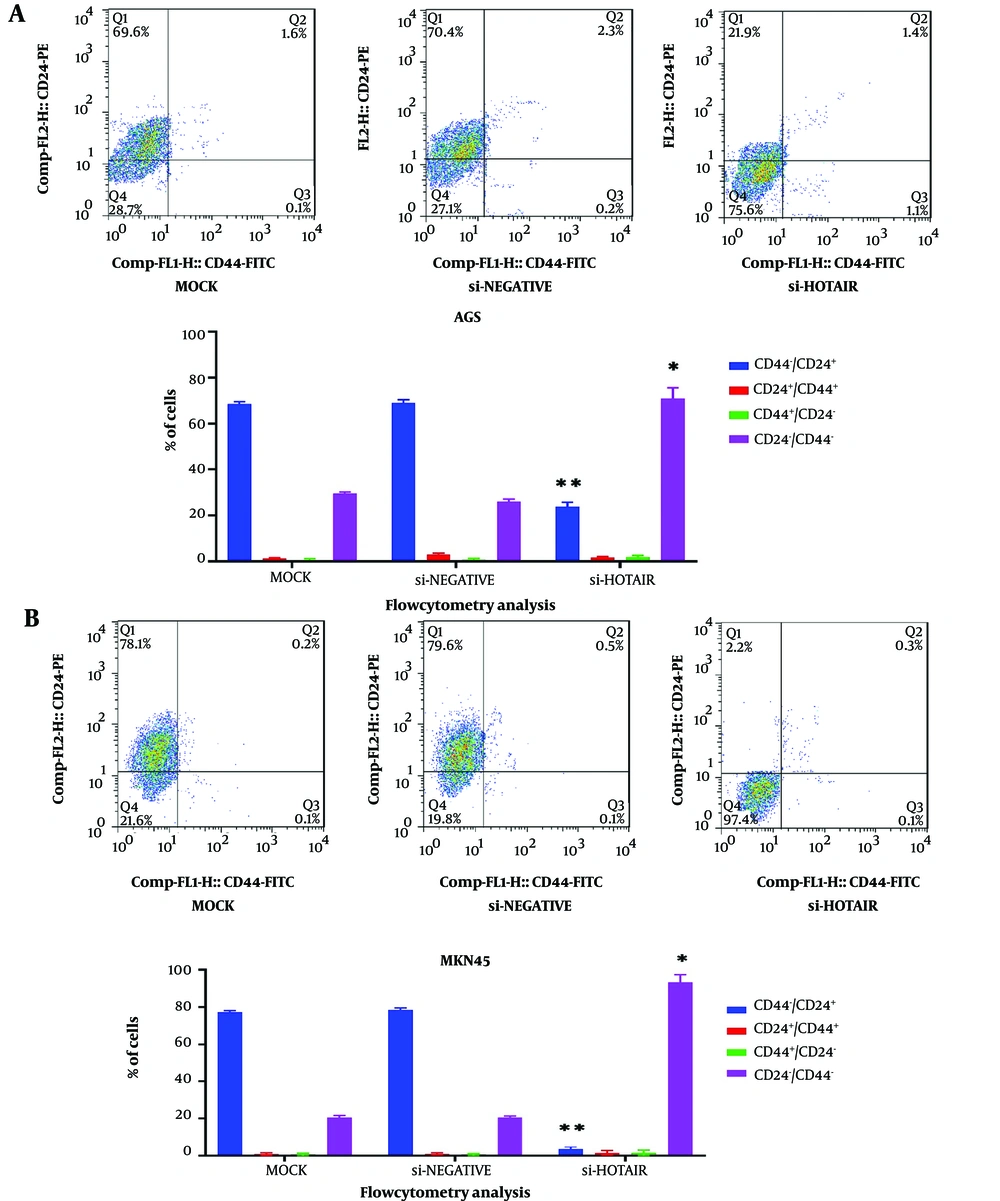
Conversely, the suppression of HOTAIR expression led to a reduction in the levels of surface CD markers, as shown in Figure 3. In conclusion, HOTAIR can amplify the characteristics of CSCs in gastric cancer cells.
Impact of HOTAIR downregulation on CD44 and CD24 Markers. Dot plots and percentages of CD44+/CD24+, CD44+/CD24−, CD44−/CD24+, and CD44−/CD24− subpopulations in A, AGS; and B, MKN45 cell lines, as determined by flow cytometry. Each experiment was conducted in triplicate for each sample (*P < 0.05 and **P ≤ 0.01).
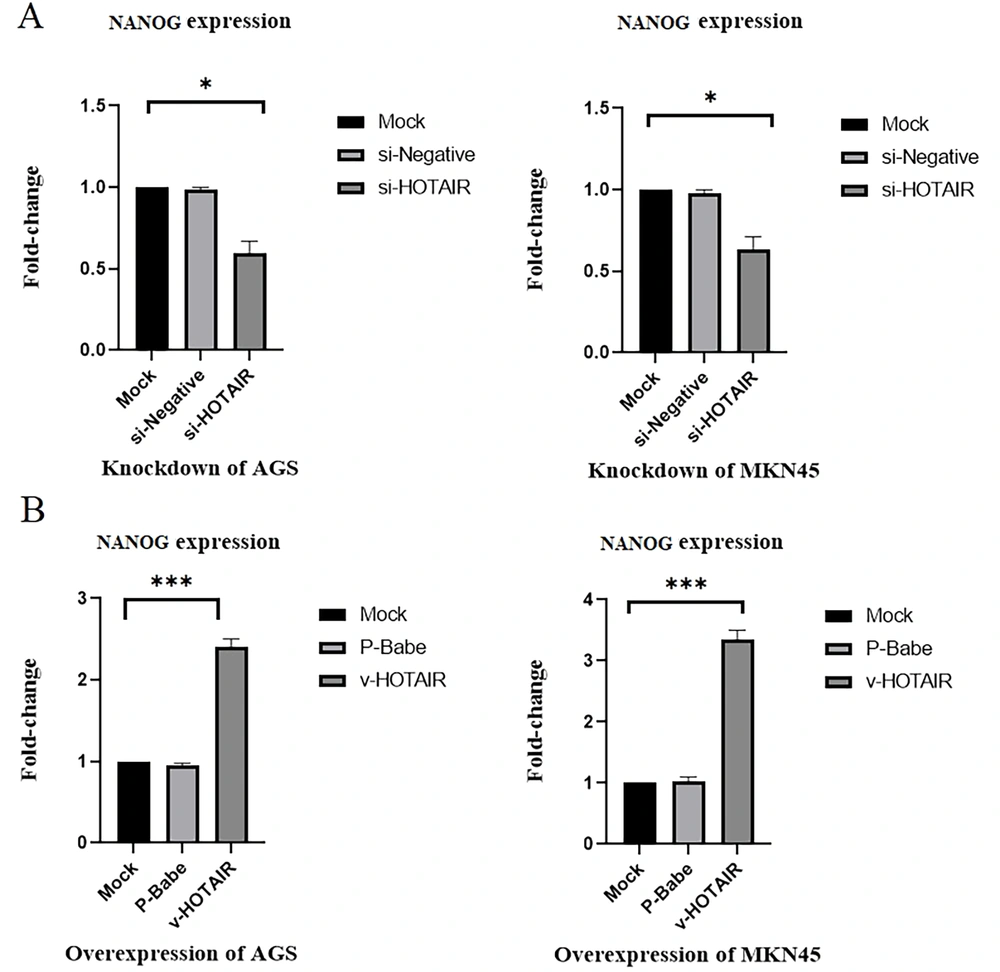
4.3. HOTAIR Expression Significantly Influences NANOG Expression in Gastric Cancer
Cancer stem cell (CSC) transcription factors, such as NANOG and SOX2, are key regulators of cell proliferation. This study examined the impact of both downregulation and upregulation of HOTAIR on cell proliferation. Quantitative Reverse Transcription PCR(qRT-PCR) analysis revealed that knockdown of HOTAIR in AGS and MKN45 cell lines significantly reduced the expression levels of NANOG (AGS = 0.4 and MKN45 = 0.37; P < 0.05; Figure 4). Conversely, overexpression of HOTAIR in these cell lines markedly increased NANOG expression (AGS = up to 2.4-fold and MKN45 = up to 3.35-fold; P < 0.001; Figure 4). Thus, HOTAIR expression appears to play a critical role in regulating stemness progression in gastric cancer.
Changes in NANOG expression levels due to HOTAIR knockdown and overexpression in gastric cancer cell lines. A, HOTAIR downregulation led to decreased NANOG expression levels (0.4 in AGS and 0.37 in MKN45). B, Upregulation of HOTAIR significantly increased NANOG expression levels (up to 2.4-fold in AGS and up to 3.35-fold in MKN45) (*P ≤ 0.05, and ***P ≤ 0.001).
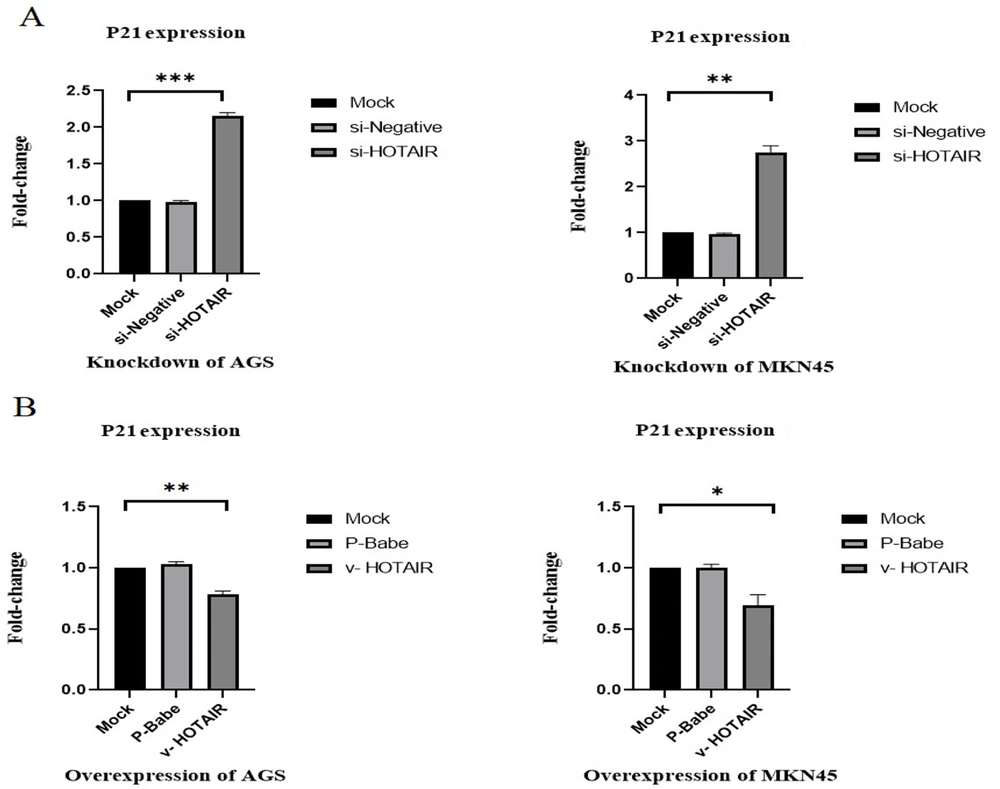
4.4. HOTAIR Modulates Gastric Cancer by Negatively Regulating Tumor Growth Suppression
Cancer fundamentally involves uncontrolled cell division. In this study, we investigated the effect of HOTAIR on P21, a key regulator of cell cycle progression at the G1 phase. Our analysis indicated that knockdown of HOTAIR in AGS and MKN45 cell lines significantly increased the expression levels of P21 (up to 2.15-fold in AGS and up to 2.7-fold in MKN45; P < 0.001; P < 0.01; Figure 5). Conversely, overexpression of HOTAIR in these cell lines notably decreased P21 expression levels (0.22 in AGS and 0.31 in MKN45; P < 0.05; P < 0.01; Figure 5).
Changes in P21 expression levels due to HOTAIR knockdown and over expression in gastric cancer cell lines. (A) HOTAIR downregulation significantly increased P21 expression levels (up to 2.15-fold in AGS and up to 2.7-fold in MKN45). (B) Up-regulation of HOTAIR significantly decreased P21 expression levels (0.22 in AGS and 0.31 in MKN45) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
5. Discussion
Long noncoding RNAs (lncRNAs) were once widely regarded as 'transcriptional noise.' However, numerous studies have now shown that lncRNAs are involved in various cellular processes, including stem cell pluripotency, cell growth, and disease pathogenesis (22). LncRNA HOTAIR is encoded by the homeobox C gene (HOXC), situated between HOXC11 and HOXC12 on chromosome 12q13.13 (23). Recent studies have highlighted HOTAIR's strong association with various types of cancer (24). For example, high levels of HOTAIR in non-small cell lung cancer (NSCLC) cell lines have been observed, and its downregulation significantly impedes proliferation, migration, and invasion by stimulating miR-217 expression (25).
The regulatory functions of HOTAIR are primarily mediated through its interactions with polycomb repressive complex 2 (PRC2) and lysine-specific demethylase 1 (LSD1) protein complexes (26). Additionally, previous research has identified numerous tumor suppressor genes, such as hMLM1, p14, p15, p16, DAP-K, THBS1, TIMP-3, RARβ, MGMT, CHFR, DCC, GSTP1, RASSF1, COX-2, APC, CDH1, CDH4, RUNX3, TSLC1, and RASSF1, that are silenced through hypermethylation in gastric cancer (27, 28). Our research focuses on examining the impact of HOTAIR on the manifestation of stem cell properties in gastric cancer cells. This includes investigating HOTAIR's influence on surface markers and the alteration of expression in key genes.
In previous studies, CD44, CD24, and CD133 have been identified as potential gastric CSC (cancer stem cell) surface markers, though there is considerable variation in the evidence supporting each of these markers (29, 30). In our study, flow cytometry was employed to determine the percentages of subpopulations within AGS and MKN45 cell lines. We observed that the CD44−/CD24+ subpopulation was present in all cell lines. A positive correlation was noted between HOTAIR overexpression and an increase in the CD44+/CD24+ subpopulation, while the CD44−/CD24− subpopulation increased in cells with HOTAIR knockdown.
As previously mentioned, CSCs are characterized by self-renewal, tumorigenicity, and chemoresistance (11). It is evident that surface CD markers play a significant role in the growth and progression of gastric cancer. For instance, CD44 and CD133 were found to be overexpressed on the surface of gastric spheroid cells, with CD44-positive cells being more tumorigenic and chemoresistant compared to CD44-negative cells (31). Our findings suggest that HOTAIR can promote stem cell-like properties in gastric cancer cells. However, the mechanisms through which HOTAIR regulates and maintains stemness CD markers are not fully understood.
Our qRT-PCR analysis indicates that the upregulation of HOTAIR significantly influences NANOG expression, whereas HOTAIR downregulation is crucial for decreasing NANOG expression. NANOG, a well-known stemness marker, has also been implicated in promoting cancer. For example, NANOG enhances cell proliferation, invasion, and CSC properties through IL-6/STAT3 signaling in esophageal squamous carcinomas (32). Various mechanisms have been proposed to regulate NANOG in gastric cancer, including a link with Helicobacter pylori infection and its influence on CSC-like features in gastric cancer (33). Previously, the role of HOTAIR in gastric cancer stemness was unclear, though it was known that targeting lncRNA HOTAIR in oral carcinoma stem cells suppresses cancer stemness and metastasis (34). Now, it appears that lncRNA HOTAIR may play a role in regulating gastric cancer stemness by modulating stemness transcription factors.
In our study, we observed a significant decrease in P21 expression upon HOTAIR upregulation. Conversely, downregulating HOTAIR proved critical for increasing P21 expression. This indicates a negative correlation between P21 expression and cell proliferation in gastric cancer cell lines. P21, a cyclin-dependent kinase inhibitor, plays a vital role in cell cycle arrest (35). The impact of HOTAIR on P21 expression in various cancers has been documented. For instance, in non-small cell lung cancer cells, cisplatin sensitivity is mediated by the upregulation of P21 when HOTAIR is inhibited (36). In breast cancer, HOTAIR indirectly regulates P21 by modulating p53 binding to the P21 promoter region, thereby reducing P21 expression (20).
Previous studies have shown that NANOG regulates the expression of cyclin D1 and c-Myc, preventing cell cycle block at the G0/G1 phase (14, 37). In contrast, P21 plays a crucial role in G1 arrest. As a result, HOTAIR can modulate the expression of both NANOG and P21, thereby influencing cell cycle regulation and contributing to the progression of cancer stemness in gastric cancer cells. However, the exact mechanism linking HOTAIR, NANOG, and P21 remains to be fully elucidated. For further analysis, animal models can provide significant insights when simulating gastric cancer and evaluating the efficacy of stem cell treatments.
5.1. Conclusions
In conclusion, our research has established that the long noncoding RNA HOTAIR plays a significant role in the regulation of stemness, cell cycle, and proliferation in gastric cancer cells. By influencing these pathways, HOTAIR promotes cancer cell growth and contributes to tumor development in patients with gastric cancer. Innovative experimental approaches are necessary to fully understand HOTAIR's mechanisms of action. Moreover, HOTAIR expression serves as a prognostic marker for cancer progression and represents a potential therapeutic target. To our knowledge, this is the first study to demonstrate the impact of HOTAIR on CD24/CD44 markers in gastric cancer. Nevertheless, further research is required to elucidate the exact mechanisms in detail. Ultimately, our study underscores the importance of understanding the complex interplay between non-coding RNAs and coding transcripts, which could prove beneficial in the development of new treatment strategies for gastric cancer.