1. Background
Artemisia sieberi (Dermeneh Dashti) is a highly aromatic shrub, serving as a prominent constituent of plant communities within Iran's dry and semi-arid steppes. Typically growing between 30 and 50 cm in height, its numerous and dense branches culminate in a mound-shaped appearance. Belonging to the Kashnikaceae family, all aerial parts of this plant, including stems, leaves, flowers, fruits, and seeds, are endowed with an intense and pervasive aroma (1).
With a bushy structure, this species holds relative fodder value and exhibits resilience in coping with adverse natural conditions, including environmental dryness, arduous climatic circumstances, and factors such as extensive and unplanned grazing. Its substantial medicinal significance is complemented by robust antioxidant attributes and rich phenolic compounds, with fresh herb exhibiting superior capacity over its two-year-old counterpart (2). This plant, standing at a height of 30 to 40 cm, features two distinct leaf types with varying divisions, wherein lower leaves showcase narrow and diminutive segments (3).
Colorectal cancer (CRC) is the third most prevalent cancer globally, constituting the principal cause of cancer-linked mortality among both men and women. While surgical interventions, chemotherapy, and radiotherapy have bolstered CRC treatment to a certain extent, disease recurrence and metastasis endure as primary determinants of CRC-associated fatalities (4).
Among the spectrum of CRC cell lines, the human colon adenocarcinoma cell line HT-29 plays a pivotal role, serving not only as a model for investigating human colon cancer biology but also as a subject of focused inquiries due to its capacity to mirror mature intestinal cell characteristics and its relevance in studies pertaining to food digestion and bioavailability (5).
The Bax protein plays a pivotal role as a key regulator in intrinsic apoptosis, while Bcl-2 exerts an anti-apoptotic influence by impeding cytochrome C release from mitochondria in response to diverse apoptotic stimuli. These genes occupy a crucial position within the mitochondrial apoptosis pathway. Given the disrupted balance between proliferation and apoptosis in cancer cells, and the imperative role of thwarting apoptosis for cell growth and proliferation, the connection between gene expression and oncogenesis underscores a crucial context for exploration within cancer research (6).
Dysregulation of apoptosis, a highly orchestrated process of programmed cell death, often arises from the alteration of various cell signaling pathways. Such dysregulation can significantly contribute to the development and progression of cancer (7).
2. Objectives
Consequently, the present study aims to investigate the impact of Artemisia sieberi plant extract on modulating the expression of pivotal genes within the apoptosis signaling pathway in the HT-29 cell line.
3. Methods
In this study, the impact of Artemisia sieberi plant extract on the colon cancer cell line HT-29 was investigated. Initially, the HT-29 cell line, sourced from the Institut Pasteur of Iran with NCBI code C135, was prepared for experimentation. The herbarium plant of Artemisia sieberi, bearing the herbarium number 85834 = TARI, was sourced from the medicinal plant garden of Iran's Forestry and Pastures Research Institute. These plants were collected from their native regions in Iran. The extraction of the plant material was conducted using the Soxhlet method. In this technique, 500 grams of plant powder were placed into a filter paper and subjected to a Soxhlet apparatus with 500 mL of extraction solvent for one hour. Subsequently, the resultant extract was concentrated using a rotary evaporator at 50 degrees Celsius and further desiccated in an oven under the same temperature conditions.
3.1. Cell Culture
The cells were cultured in RPMI1640 culture medium (Biosera; USA) supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS), and maintained at 37°C with 5% CO2. For cell counting, 50 µL of the cell suspension was initially mixed with 50 µL of 0.25% trypan blue. After 1 minute, the mixture was transferred to a Neobar slide and then examined under a microscope. Specifically, 20,000 cells were seeded per well of a 96-well plate, 500,000 cells per well of a 6-well plate, and 1,500,000 cells were seeded in a 25 cm² flask.
3.2. Cell Treatment
After the cells were initially seeded, a 24-hour incubation period was provided for cellular adherence to the plate surface. Subsequently, the supernatant culture medium was discarded, and a phenol red-free medium supplemented with 5% FBS was introduced to the cells, allowing an additional 24-hour period for cellular acclimatization to the modified environment. Finally, the supernatant was removed, and the cells were exposed to varying concentrations of the probiotic extract (1, 10, 100 µM). To administer the treatment, the substance was initially dissolved in DMSO to create a 0.1 M stock solution. Subsequent dilutions were prepared to obtain concentrations of 1, 10, and 100 µM, which were then added to the cells. Following a 48-hour exposure to the specified compounds, the cells underwent subsequent testing procedures.
3.3. MTT Assay
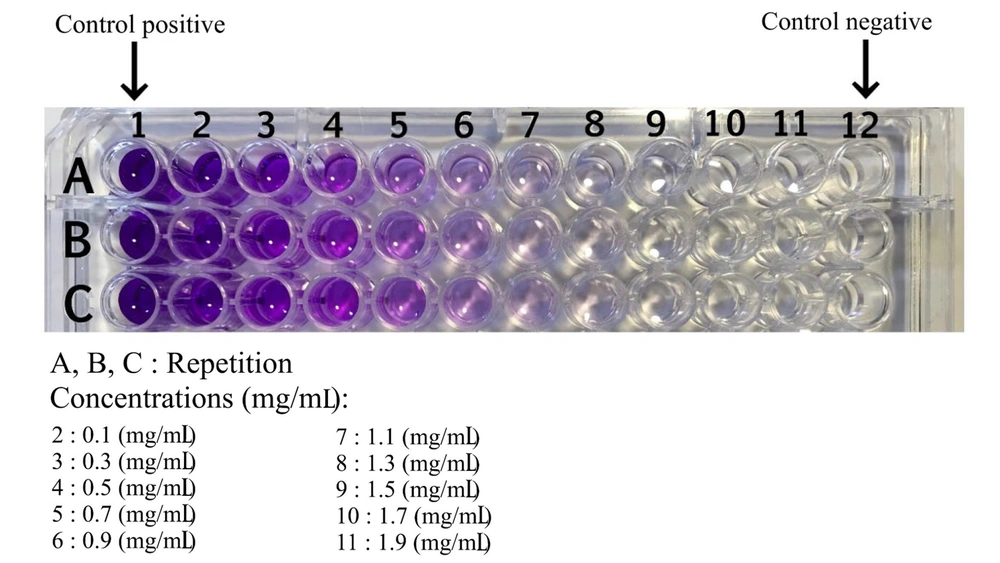
The MTT assay was employed to assess cell viability at varying doses. Cells were initially seeded in a 96-well plate and subsequently treated with concentrations ranging from 0.001 to 100 µM (each concentration in six wells) for a duration of 48 hours. Following the incubation period, the culture medium was removed, and 100 µL of culture medium containing 10% (w/w) MTT solution was added to the cells. The MTT solution was prepared by dissolving 10 mg of MTT in 5 mL of PBS, followed by filtration using a 0.2 µm filter, and stored away from light at a temperature of 4°C. The plate was then placed in the incubator and incubated with MTT for 3 hours. Subsequently, the supernatant was carefully aspirated, and 200 µL of DMSO was added to each well to dissolve the formazan crystals, resulting in a uniform blue solution. The absorbance of this solution was read at 570 nm using an ELISA reader. Each experiment was replicated three times.
3.4. Real-time Polymerase Chain Reaction
The RT-PCR procedure was carried out using a Rotor-Gene Q machine. The primer design was executed using Oligo7 software, with details presented in Table 1. To ensure primer specificity, the designed sequences underwent BLAST analysis on the NCBI website. Following analysis, a specific volume of DEPC water was added to each primer to achieve a final concentration of 100 pmol, and the primers were then stored at -20°C. Subsequently, the expression levels of GAPDH, Bax, and Bcl-2 genes were assessed using the real-time PCR machine. Notably, the GAPDH gene served as the internal control in this investigation.
| Location | Sequence (5'-3') | Strand | Tm°C | Purification |
|---|---|---|---|---|
| Primer Bcl-2: Amplicon size = 119 bp | ||||
| 951553 | GTGGATGACTGAGTACCT | Forward | 53.69 | DESALT |
| 951648 | CCAGGAGAAATCAAACAGAG | Reverse | 55.25 | DESALT |
| Primer Bax: Amplicon size = 152 bp | ||||
| 456312 | CTACAGGGTTTCATCCGA | Forward | 53.69 | DESALT |
| 478926 | CCAGTTCATCTCCAATTCG | Reverse | 54.51 | DESALT |
| Primer GAPDH: Amplicon size = 102 bp | ||||
| 154148 | CAGGTCAATCTTCACTTC | Forward | 63.3 | DESALT |
| 154228 | ATGATCCACAGTAGCG | Reverse | 61.3 | DESALT |
4. Results
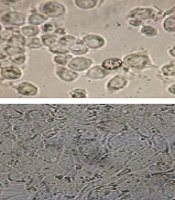
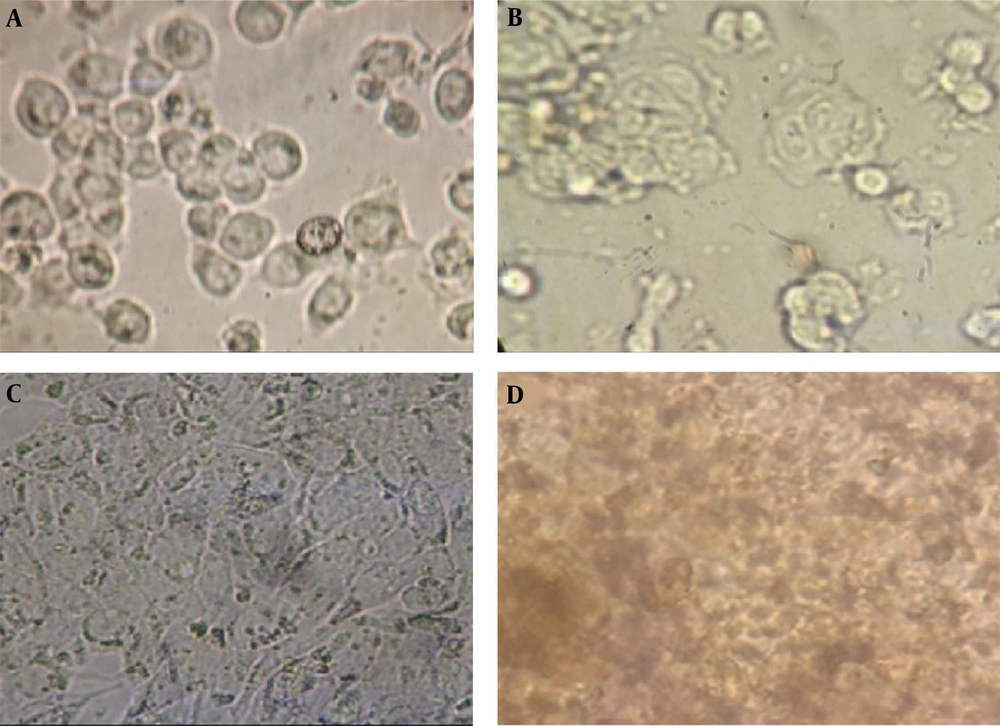
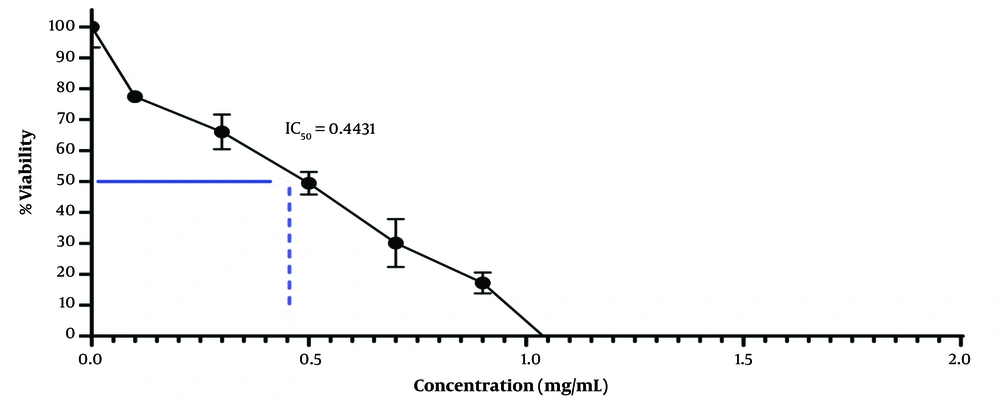
The HT-29 cell culture, subsequent to cellular growth and differentiation, underwent treatment with the extract of Artemisia sieberi (Figure 1). The MTT method was employed to evaluate the cytotoxicity of the plains sage extract. Results demonstrated that an increase in the concentration of the studied extract led to a dose-dependent decrease in cell viability. Notably, the IC50 values for the plains herb extract were determined to be 0.44 μg/mL (Figure 2).
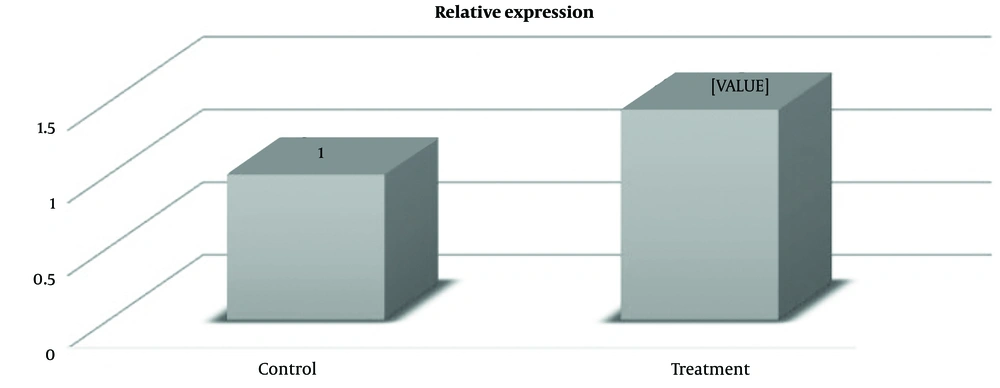
Fold change represents an effective means to articulate the magnitude of gene alterations in one sample versus another. It reflects the proportional difference in gene expression levels between treatment and non-treatment samples. In this investigation, the Bcl-2 gene exhibited a fold change of 0.45 in cells treated with the plains herb extract. This signifies a 0.45-fold increase in gene expression compared to the untreated cells, as denoted by a P-value of 0.01 (Figure 3). Moreover, research findings revealed that the expression level of the Bax gene in cells treated with the plains herb extract amounted to 1.48, indicating a 1.48-fold increase in gene expression relative to the non-treated cells, with a P-value of 0.001 (Figure 4).
A, Photo presents the HT-29 cell line before; and after; B, treatment with the extract of the Artemisia sieberi. Additionally, the figure depicts the untreated cells at the time of testing in 42-well plates; C, differentiated cells for cell treatment; and D, cells treated with the extract Artemisia sieberi for 42 hours.
5. Discussion
Cancer ranks among the leading causes of mortality worldwide. According to WHO estimates, the year 2030 is projected to witness over 21 million new cases of cancer and more than 13 million cancer-related deaths globally (8). In Iran, colon cancer is one of the most prevalent types of gastrointestinal cancer, ranking as the third most common cancer in Iranian men and the fourth most common in women (9).
Recent research has spotlighted Artemisia sieberi, particularly focusing on its antioxidant content over time. Medicinal plants serve as valuable sources of biologically active phytochemical compounds that combat free radicals as antioxidants. This investigation explored various extraction methods pertaining to phytochemistry, essential oil yield, and extractions from fresh and two-year-old Artemisia sieberi. Results revealed that the antioxidant efficacy of methanolic and ethanol extracts surpassed that of essential oils, attributed to differing phenolic compounds. Notably, the fresh herb displayed higher antioxidant potential and phenolic compounds compared to the two-year-old herb (10, 11).
Pro-apoptotic proteins within the Bcl-2 family influence the mitochondrial membrane to facilitate permeabilization and the release of cytochrome C and ROS, pivotal signals within the apoptotic cascade (12). BH3-only proteins activate these pro-apoptotic proteins while being inhibited by Bcl-2 and its relative Bcl-Xl (13).
The findings from Sattari and Ahmadizadeh (14) in 2017, titled "Evaluation of Bcl-2 and akt1 gene expression in adjacent culture of HT-29 colon cancer cells," revealed that the plains sage extract reduced the expression of the Bcl-2 gene in treated strains compared to non-treated ones by 2.3 times. MTT testing indicated that an OD concentration of 0.05 exhibited the highest lethality within a 24-hour span. The researchers concluded the potential for developing a novel therapeutic solution with heightened efficacy, minimal side effects, biological safety, and cost-effectiveness derived from the plains herb extract or as an adjunct treatment in alignment with the body's natural flora for colon cancer treatment and prevention. These findings align with the outcomes of the present study (15).
In Zibasaz Talebi and Ahmadizadh's 2020 study, which explored the effects of side-by-side cultivation of plains heather extract on the expression levels of Bcl-2 and Bax genes in cancer cells, it was uncovered that the Artemisia sieberi extract elevated the expression of Bax and Bcl-2 genes while inducing apoptosis in the HT-29 cell line. Hence, further research could explore the potential use of Artemisia sieberi extract in colon cancer treatment and prevention, aligning with the outcomes of the present research (16).
The research presented illustrates a comprehensive exploration of the potential benefits of Artemisia sieberi in cancer treatment, emphasizing its role in modulating gene expression and apoptotic pathways. One limitation of this study is the lack of in vivo studies to validate the effects observed in cell culture settings. Animal studies or clinical trials would provide further evidence of the efficacy and safety of Artemisia sieberi extract. Additionally, understanding the long-term effects and potential side effects of using Artemisia sieberi extract in cancer treatment is crucial for assessing its overall safety and efficacy.
5.1. Conclusions
Based on the low toxicity exhibited by the Artemisia sieberi extract in the MTT test, coupled with its apparent impact on enhancing gene expression associated with hindering apoptosis in intestinal cells, Artemisia sieberi extract holds potential as a therapeutic supplement for both the prevention and treatment of gastrointestinal cancers. Native plants, leveraging their inherent properties, offer promise in formulating new therapeutic solutions that possess potent impact, minimal side effects, biological safety, and lower costs. These plant-derived compounds could serve as beneficial adjunctive treatments, in harmony with the body's natural flora, in the endeavor to prevent and address the challenges posed by colon cancer.