1. Background
The survival of human life hinges on the foundational aspects of learning and memory. Learning represents multiple changes that occur in behavior as a result of experience, and memory, as the end product of learning, is vital for retaining those learned experiences (1). A myriad of human diseases can induce progressive damage to cognitive functions, with Alzheimer's disease being a notable neurodegenerative condition. This is due to the involvement of cholinergic, catecholaminergic, serotonergic, GABAergic, histaminergic, and glutamatergic systems (2). These systems typically contribute to various regions in the brain associated with learning, memory, and long-term potentiation (LTP). The hippocampus, an integral part of the prefrontal cortex in the mammalian brain, is considered the main structure for spatial learning and memory consolidation (3).
As the N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors are activated in the hippocampus, an inflow of extracellular calcium ions through these receptors occurs across the postsynaptic terminal membrane, providing the key element for LTP induction. Moreover, the advanced function of NMDA receptors enhances learning and memory, resulting in an increase in calcium during synaptic transmission (4).
Androgens, particularly testosterone, also play a specific role in recognition and memory. Low levels of testosterone and its elimination can trigger memory disorders and augment neurodegenerative diseases and brain injuries, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (5).
Given that androgen receptors (ARs) can be found in different regions of the brain, primarily the hypothalamus, thalamus, hippocampus, amygdala, basal ganglia in the frontal brain, and cerebral cortex, they are well differentiated (6). Cholinergic basal forebrain neurons require estrogen to enhance the acquisition rate in simple spatial learning during T-maze challenges. Estradiol (E2) selectively regulates agonist binding sites on the NMDA receptor complex in the hippocampal CA1 region (7). Estrogen administration has been reported to increase dendritic spine density in the hippocampal CA1 region in brain slices from male rats (8).
Considering the benefits of cumin (Cuminum cyminum L.), also known as caraway in the family Apiaceae, several medicinal properties have been documented for this plant. These include healing the stomach, alleviating flatulence (9), acting as an anti-cancer agent, inhibiting platelet aggregation, possessing anti-epileptic and anti-convulsant properties (10), exhibiting anti-diabetic characteristics (11), enhancing milk secretion and estrogenic activities (12), and having a strong antioxidant role (13).
Despite the documented benefits of cumin, further research is needed to explore its other uses.
2. Objectives
This study investigated the effect of cumin hydroalcoholic extract (HAE) on learning and memory in gonadectomized (GDX) male Wistar rats.
3. Methods
To prepare the cumin hydroalcoholic extract (HAE) for this study, cumin seeds were purchased and identified by the herbarium affiliated with Islamic Azad University (IAU), Qom branch, Qom, Iran. Initially, the Soxhlet apparatus temperature was set at a mild level of 25°C, and then 300 ml of 70% ethanol was added to the balloon. Afterward, 40 g of cumin seeds were powdered, placed into a cloth bag, and positioned in the balloon. As the mixture was heated, the solvent gradually evaporated over 24 hours, allowing the active plant ingredients to be extracted into the solvent through repeated cycles. The extract-containing solvent was then filtered and dried in an oven at a temperature of 38°C. The resulting shiny powder extract was stored in a dark container, covered with aluminum foil, and kept in the freezer.
3.1. Animals
For this study, 48 adults male Wistar rats, weighing 250 ± 50 g, were purchased from the Pasteur Institute of Iran, Karaj, and then transferred in special cages to the animal house of IAU, Qom branch, Qom, Iran. The rats were provided with sufficient water and pellet food ad libitum, except during the experiment. To reduce stress and acclimate them to the new environment, the rats were transferred to the animal house one week before the study. The breeding room was maintained at a temperature of 25°C, with a 12-hour light and 12-hour dark cycle, relative humidity of 40 - 60%, and no noise pollution.
3.2. Grouping
A total of eight groups were created for this study: A normal group treated with dimethyl sulfoxide (DMSO) (0.5 cc), three normal groups receiving cumin HAE doses of 25, 50, and 100 mg/kg, a GDX group treated with DMSO (0.5 cc), and three GDX groups receiving cumin HAE doses of 25, 50, and 100 mg/kg.
3.3. Passive Avoidance Learning (PAL) Measurement
A Shuttle box (manufactured in Iran), consisting of two plastic compartments, was used to measure learning and memory in the adult male Wistar rats in this study. The light compartment measured 20 cm × 20 cm × 30 cm, and the dark compartment measured 20 cm × 20 cm × 30 cm. There was a guillotine-style door between the compartments, measuring 8 cm × 8 cm, which could be opened and closed with a wire. The floors of both compartments were covered with stainless steel bars, each 2 mm thick and 1 cm apart. The dark compartment floor could be electrified by connecting it to a power supply, with adjustable electricity duration and volume set to 2 mA, 5 s, and 50 Hz for this experiment. The PAL measurement phases included acclimatization (adaptation), acquisition (learning), and recall (testing) (14).
3.4. Acclimatization (Adaptation) Phase
To acclimatize the rats to the experiment site, they were placed in the laboratory environment 30 minutes prior to the experiment. During this phase, each rat was first positioned in the light compartment of the Shuttle box. After 10 seconds, the guillotine-style door was opened, and the step-through latency (STL) was recorded. Once the rat completely entered the dark compartment, the door was closed, and the rat was removed from the box and returned to its cage. Due to the inherent tendency of rats to enter dark spaces, most rats entered the dark compartment of the box after a short while. Any rat that took longer than 120 seconds to enter the dark compartment was excluded from the experiment.
3.5. Acquisition (Learning) Phase
In this phase, the rats were placed in the light compartment of the Shuttle box half an hour after the intraperitoneal (IP) injections. After 10 seconds, the guillotine-style door was opened, and the step-through latency (STL) was recorded. Upon entering the dark compartment, the guillotine-style door was closed, and a foot shock (2 mA, 5 s, and 50 Hz) was administered. Due to the closed roof of this compartment, the rats could not avoid the electric shock, making it an unavoidable shock. The rat was removed from the box and transferred to its cage 20 seconds after the end of the shock. This procedure was repeated 2 minutes later. If the rat entered the dark compartment again, another shock was administered. This process was repeated up to a maximum of three times for each rat.
3.6. Recall (Testing) Phase
One day after the acquisition (learning) phase, the recall (testing) task was conducted. First, the animal was placed in the light compartment of the Shuttle box. After 10 seconds, the guillotine-style door was opened, and the step-through latency (STL) was recorded as the delay time and an index to check memory. In this phase, no foot shock was applied, and both the STL and the total time in the dark compartment (TDC) were recorded. This phase lasted 600 seconds. A rat that remembered being shocked in the dark compartment and avoided entry (passive avoidance learning, or PAL) showed a significant increase in STL compared to the acquisition (learning) phase, indicating improved memory. To measure the effect of the extract, it was also injected intraperitoneally (IP) half an hour before the experiment.
3.7. Study Indices
Two indices were employed to improve accuracy in evaluating the effect of the treatment, as follows:
- STL (Step-Through Latency): This represents the time (in seconds) taken by the animal to enter the dark compartment of the box from the light one.
- TDC (Total Time in Dark Compartment): This represents the time the animal spends in the dark compartment of the box during the experiment, with a maximum of 600 seconds.
3.8. GDX
Before removing their testes, the rats were anesthetized with intraperitoneal (IP) injections of ketamine (75 mg/kg) and xylazine (25 mg/kg). The midline hairs on the ventral surface of their scrotum were shaved and disinfected with alcohol. An incision of 1.5 cm was made along the scrotum skin with sharp, disinfected surgical scissors. The transparent membrane around the testes (tunica) was grasped with sterile forceps and cut to release the organs. The vas deferens and surrounding fat tissue were then tied twice with absorbable suture threads. The vas deferens between the knot and the testis were cut with surgical scissors, and the testis was removed. The same procedure was repeated for the other testis. Once both testes were removed, the skin incision was stitched two or three times, and betadine solution and penicillin powder were applied to disinfect the surgical site. Finally, the animals were placed in alcohol-disinfected cages to regain consciousness. The GDX rats were kept in individual cages for one week and then underwent experiments after a two-week recovery period.
3.9. Data Analysis
The data included the STL and TDC duration one day after the experiment in the normal and GDX groups. Using IBM SPSS Statistics software (version 19), a one-way analysis of variance (ANOVA) and Tukey's test were applied to analyze the data, with P < 0.05 considered the level of significance.
4. Results
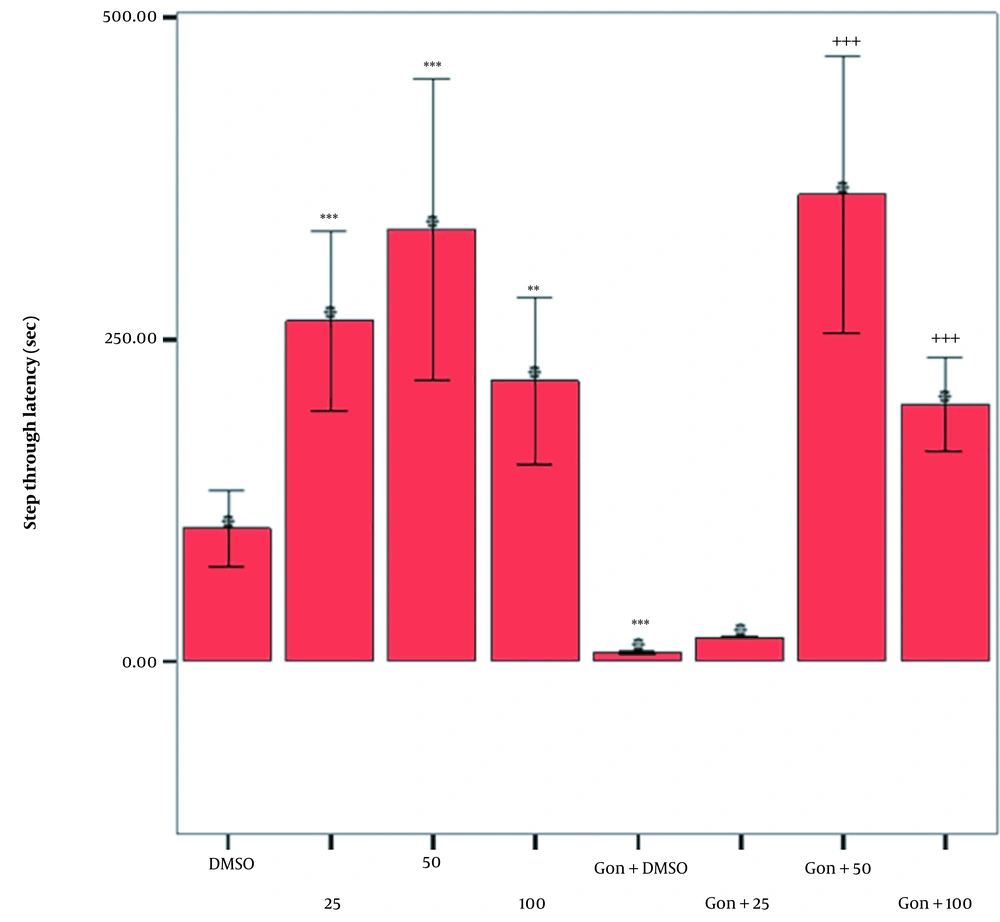
The normal and GDX male Wistar rats received DMSO and different doses of cumin HAE through IP injections half an hour before the recall (testing) phase in the PAL. On the experiment day, the STL was recorded. According to Figure 1, the study results demonstrated a significant increase in STL after administering cumin HAE doses of 50 and 100 mg/kg in the GDX rats, compared to the GDX group treated with DMSO (P < 0.001). Additionally, the GDX rats treated with DMSO showed a significant decrease in STL compared to the normal group receiving DMSO (P < 0.001).
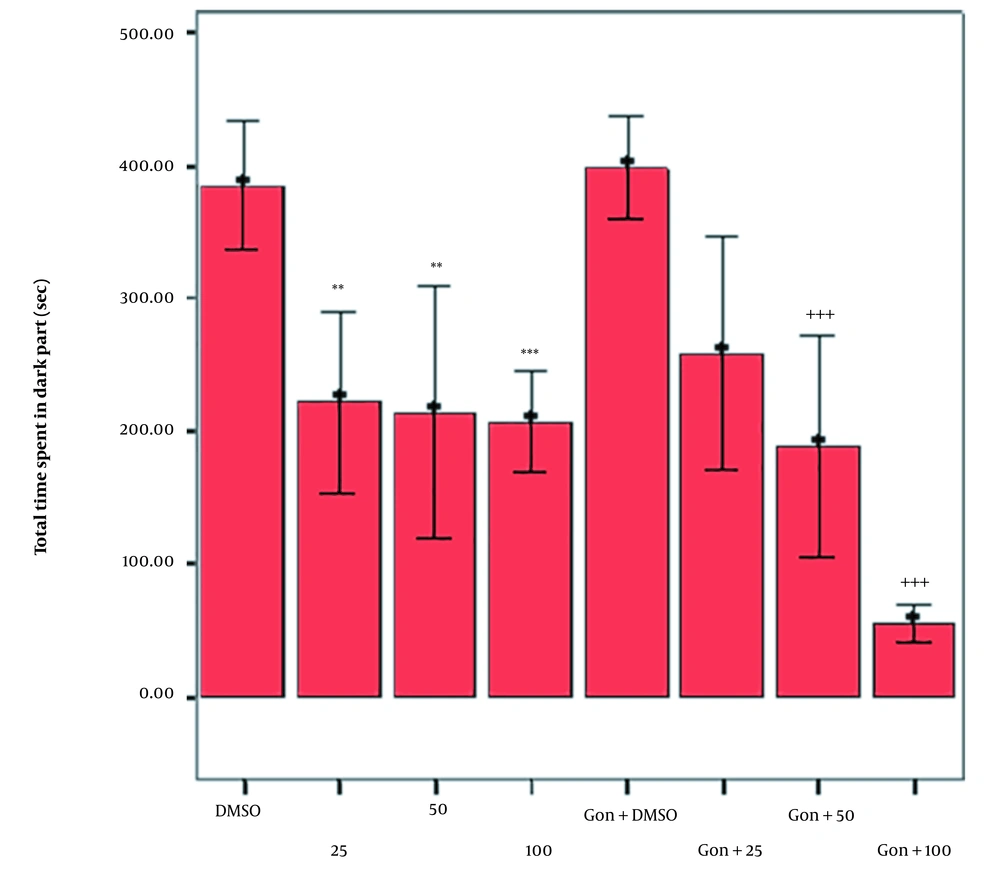
The normal and GDX male Wistar rats were also treated with DMSO and different doses of cumin HAE through IP injections half an hour before the recall (testing) phase in the PAL. On the experiment day, the TDC was recorded, as illustrated in Figure 2. The results showed a significant decline in TDC in the GDX male Wistar rats receiving cumin HAE doses of 50 and 100 mg/kg compared to the GDX group treated with DMSO (P < 0.001). Notably, the GDX rats taking DMSO did not present a significant difference in TDC (Figure 2).
5. Discussion
Cuminum cyminum Linn. (Apiaceae), commonly known as cumin, is a popular spice that has been utilized for centuries for its medicinal properties in alleviating a range of symptoms. Although the effects of this plant on anxiety (15), memory loss and stress (16), and age-related neurological disorders (17) have been examined in previous studies, the current research is the first report on the hydroalcoholic extract of cumin and its impact on passive avoidance learning (PAL) in gonadectomized (GDX) male Wistar rats. The study results indicated that GDX reduced PAL, while cumin HAE doses of 50 and 100 mg/kg in the GDX male Wistar rats augmented its incidence.
Neurohormones like estrogens and androgens are synthesized within the hippocampus, influencing learning and memory. The increased receptor density and simultaneous testosterone production in key centers linked to cognitive functions, particularly the hippocampus, underscore their vital roles in these processes (18). It seems that the effects of testosterone and androgen occur directly and indirectly once dihydrotestosterone (DHT) and E2 are transformed (19).
Since testosterone is converted into E2 by aromatase, the memory-enhancing effects of testosterone could stem from the conversion of these substances into E2 within the hippocampus and the amygdala. Therefore, the mechanism of testosterone in the hippocampus in men parallels that of E2 in women (20). As reported in some experiments, administering estrogen to male animals or estrogen to females has thus far assisted learning (21).
The signaling pathways and gene expressions regulated by estrogen include the activation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB), NMDA receptors, glutamic acid decarboxylase (GAD), choline acetyltransferase (ChAT), synaptic density, and related proteins. It is evident that rapid improvements in cognition can be triggered by the activation of nuclear estrogen receptors (ERs) in the membrane, particularly through the mitogen-activated protein kinase (MAPK) pathway at specific neural sites (22). For example, estrogen enhances performance in tasks such as inhibitory avoidance and target recognition within 4 hours of treatment (23).
Using a proof model, these post-learning outcomes have been attributed to estrogen's impact on memory and the transfer of prior information to long-term memory, as validated by McGaugh (24). Considering estrogen's actions in various nervous systems and its effects on the relative use of memory systems and the length of learning, estrogen facilitates cognitive organization. Its effects on neurotransmitters, such as acetylcholine, show a mechanism by which estrogen coordinates learning and memory by regulating other inhibitory and excitatory processes (20). In fact, the cholinergic system is activated by estrogen in brain regions critical for memory, such as the basal forebrain containing acetylcholine and the frontal cortex (25). Although the effects of this hormone are not fully clear in daily routines, they become apparent during old age or episodes of forgetfulness. Estrogen treatment improves working or short-term memory, enhances memory organization for better executive functions, and leads to cortical integration (26).
In the present study, GDX decreased learning and memory, likely through the mechanisms mentioned above.
Notably, a wide variety of plants produce substances called phytoestrogens that mimic or interfere with the action of estrogen in the body. These compounds include several groups, such as sterols, lignans, isoflavonoids, coumestans, and lactones of some flavonoids and phytosterols, which are the strongest and produce estrogen-like effects (27).
Estrogen, flavonoid, and apigenin are also present in aromatic plants such as cumin. Cumin contains lignans, which are the main compounds in the phytoestrogen family. These relatively simple polyphenolic chemicals found in plants are similar to estrogen. Their antioxidant properties prevent the pre-oxidation of fats and the production of harmful compounds such as radicals and free fatty acids (FFAs) (28).
In general, the positive effects of cumin HAE on memory observed in the present study could be attributed to its ability to elevate acetylcholine levels in the brain through its terpenoids. The cholinergic system, which boosts acetylcholine in the brain, correspondingly improved memory.
Considering the presence of arginine in cumin, as a precursor contributing to the synthesis of nitric oxide (NO), memory consolidation was facilitated. This corrected memory impairment induced by NO synthase inhibitors and returned it to its normal state. The resulting NO, produced as nerve mediators in neurons, could additionally play a leading role in long-term potentiation (LTP), memory, and learning (28). Therefore, it is possible that cumin boosted memory in this way.
5.1. Conclusions
Based on the study results, cumin HAE helps reinforce learning and memory in GDX male Wistar rats, likely due to the phytoestrogenic properties of the cumin ingredients and their effects on the cholinergic system. However, the specific processes and active substances involved in enhancing learning and memory have yet to be thoroughly defined. Consequently, additional biochemical and pharmacological research is necessary.