1. Background
There are many strategies to enhance the expression of foreign genes in transgenic plants. To achieve optimal gene expression, it is essential to construct an expression cassette incorporating the best elements influencing gene expression. Various elements affect foreign gene expression in transgenic plants, including powerful promoters. Promoters are classified into three classes, one of which is constitutive promoters. For example, the 35s CaMV and patatin class I are constitutive promoters. Another critical element affecting foreign gene expression in transgenic plants is enhancers.
In this project, we designed an expression cassette for the expression of HBsAg in transgenic potato plants, as described below (1, 2) (Figure 1).
On a global scale, hepatitis B virus (HBV) infection is likely the most significant cause of persistent viremia in humans, with current estimates indicating approximately 300 million carriers worldwide. The first commercially available recombinant HBsAg vaccine was produced in yeast. While this vaccine is effective and elicits a protective immune response, its cost is prohibitive for many developing countries. Expression of HBsAg in transgenic tobacco was first achieved by Mason et al. in 1992, and the immunogenicity of this protein in mice has been demonstrated. However, a major disadvantage of using plants for the production of heterologous proteins is their low productivity. Several approaches can be used to increase expression, such as determining the plant codon preference for the gene of interest, deleting unstable mRNA motifs, and using 5' and 3' flanking regulatory sequences (3).
2. Objectives
In our study, we aim to use different combinations of constitutive (pCaMV) and organ-specific (Patatin class I) promoters with the tobacco etch virus (TEV) translational enhancer to increase the expression level of HBsAg in transgenic potato plants.
3. Methods
All of the molecular cloning methods were done according to Sambrook et al. in molecular cloning protocols. Agrobacterium strain pGV3850 cells were transformed by direct method with the recombinant binary vector prepared from an E. coli clone (4). PCR analysis: The presence of the HBsAg gene in Agrobacteria was checked by PCR. The two primers are located at 261 and 724 nucleotides downstream from the ATG start codon of HBsAg. The sequence of the upper primer is 5’-TGTCTAGAAACATCCAATGACATAGGCCAA-3' and for the lower is 5'-CTCTTCATCCTGCTGCTATGCCTCAT-3'. The primers specifically amplify a 463 base pair DNA fragment. Each PCR amplification was carried out in a 25 μL reaction volume containing 0.5 units of Taq DNA polymerase, each primer at 0.5 μM, each deoxyribonucleoside triphosphate at 0.5 mM, 1.5 mM MgCl, 10 mM Tris-HCl, pH 8.3, and 16 mM (NH4)2SO4. After a hot start for 3 minutes at 94°C, amplification was carried out for 30 cycles in a DNA Thermal Cycler (Pharmacia-LKB) using a denaturation temperature of 93°C for 1 minute, an annealing temperature of 55°C for 1 minute, and an extension temperature of 72°C for 1 minute. The amplification products were visualized by UV after agarose gel electrophoresis (5).
Plant transformation: Agrobacterium and potato plant transformation was done according to Ishida et al. 1989. Plants that developed roots on the selection medium were assayed for the expression of HBsAg.
Analysis of HBsAg expression in plants: Plant tissue extracts were obtained according to Mason et al., 1992, and the supernatant was checked by ELISA for the presence of HBsAg (6).
ELISA test: Microplates containing rabbit polyclonal antibody against HBsAg from human serum were used for detection of HBsAg in plant tissue antibody extracts and developed by monoclonal antibodies against the "a" determinant of HBsAg conjugated with peroxidase. The concentration of HBsAg was determined against a standard curve of recombinant hepatitis B vaccine. Total protein concentration in the plant extract was determined according to Lowry (7).
4. Results
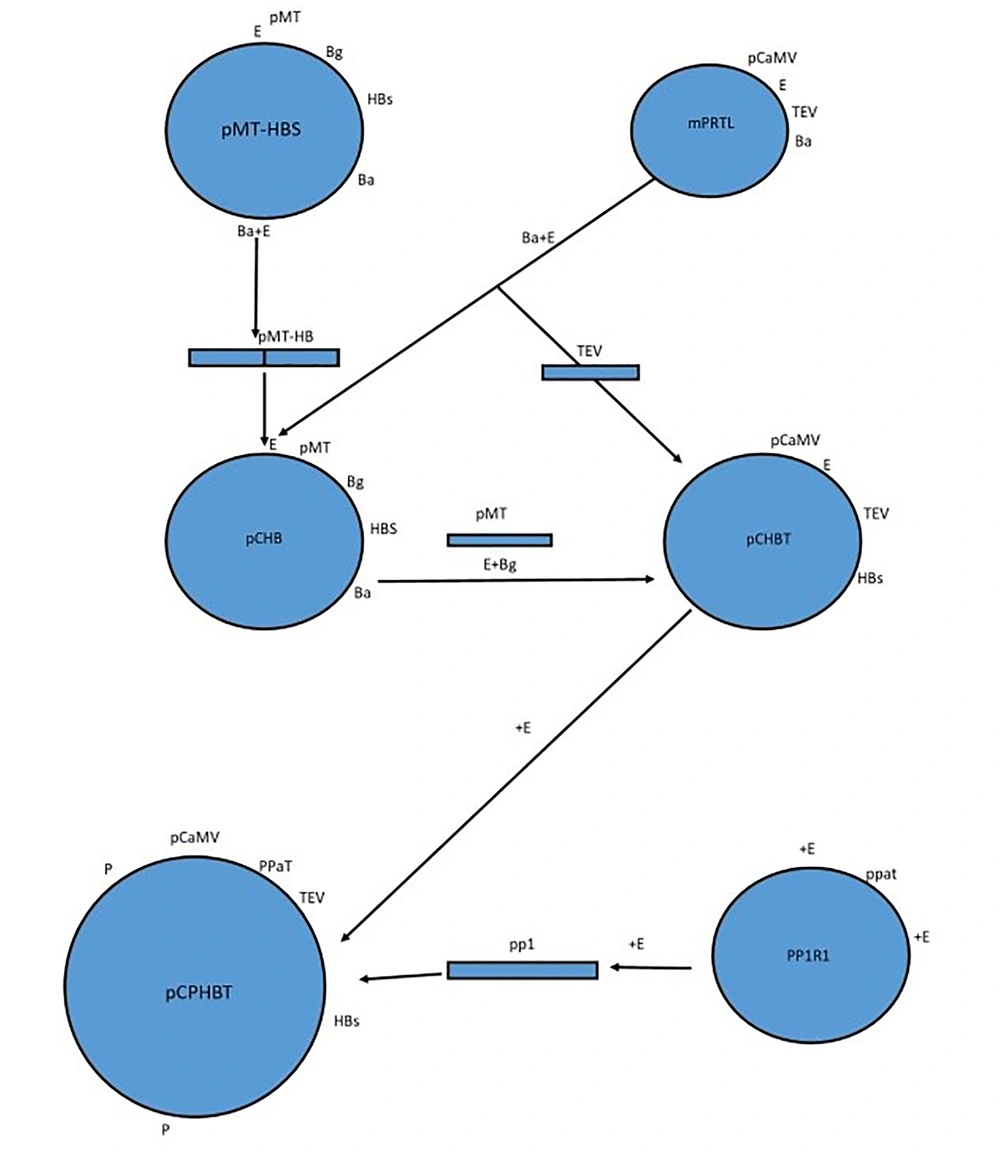
The HBsAg expression cassette with TEV leader and Patatin promoter was prepared in several steps. First, we constructed the expression cassette containing the pCaMV promoter and TEV leader. We then used this plasmid to add the Patatin promoter. We used mPRTL for obtaining the TEV leader, PPIRI for the Patatin promoter, and PMT-HBs for the HBsAg gene. It is interesting to note that all efforts to obtain the HBsAg by BamHI-BglII digestion and inserting it into the BamHI site downstream of the TEV leader sequence were unsuccessful, so we had to design another cloning strategy as follows: The mPRTL plasmid contains the 35S CaMV dual-enhancer promoter followed by the TEV leader and polyA signal (8). This plasmid is constructed for the enhanced production of fused heterologous proteins in plants. This plasmid was digested by EcoRI-BamHI to obtain the TEV leader sequence (151bp), then both plasmids with and without the TEV leader were used. The sequence coding for HBsAg subtype ay (9) was excised from PMT-HBsAg as an EcoRI-BamHI (2101bp) fragment and cloned into the modified PRTL plasmid from which the TEV sequence was already excised to produce pCHB (Figure 2).
The pCHB plasmid was digested by BglII and EcoRI to remove the PMT sequence, which was not needed in this study, and the TEV leader sequence was inserted instead to produce pCHBT. The Patatin class I promoter was obtained by EcoRI digestion (1822 bp) from PPIRI. The isolated band containing the promoter was inserted into pCHBT. The orientation of the gene in the newly produced plasmid (pCPHBT) was checked by restriction digestion analysis. The derived chimeric HBsAg gene was then excised as a PruII fragment and cloned into the multiple cloning site of the binary vector (Bin19). The plasmid structures and the presence of the HBsAg coding region were verified by restriction enzyme digestion. After Agrobacterium transformation by Bin19, amplification of a 450 bp PCR band showed that the needed sequence was present in Agrobacteria (10).
Microtuber discs were co-cultivated with Agrobacteria, and three shoots were grown and regenerated on selective media. As shown in the table, the concentration of expressed proteins tested by ELISA was 0.035%, 0.04%, and 0.05%. As mentioned before, there are several approaches to increase the expression level of heterologous genes in transgenic plants. One approach is utilizing the 5' untranslated region of the TEV translational enhancer to increase the level of protein production from the same amount of mRNA. This is the first report of the use of the TEV leader with the Patatin promoter. Previous works have shown that the maximum level of S antigen found in both transgenic tobacco and potato tubers with the pCaMV promoter without the TEV leader is near 0.01% of total soluble protein (6, 11), and for the Patatin promoter, it is 0.015% of total soluble protein in tubers (our unpublished data). Mason et al. used the pCaMV promoter along with the TEV sequence, but the production level was again about 0.01%. Our results showed that the highest concentration is 0.05% of tuber total protein (11, 12) (Table 1).
5. Discussion
According to the studies, the expression level of plants transformed by constructs containing the TEV leader sequence is 4 to 5 times higher compared to plants containing constructs without the TEV leader sequence. Studies on the utilization of these promoters and the TEV leader with the Norwalk virus capsid protein (NVCP) yielded similar results, showing a higher expression of NVCP with the Patatin promoter (0.37%) in potato tubers compared to 0.23% in tobacco leaves with the pCaMV promoter. This indicates that higher production of the antigen is possible with the Patatin and TEV leader combination compared to the pCaMV promoter. The authors confirmed that the higher production of the Norwalk virus capsid antigen compared to HBsAg is due to the nature of the antigen itself (11, 13, 14).
Our results, along with the latter report, suggest that there could be a synergistic effect with the Patatin promoter and TEV leader in comparison to pCaMV and TEV in potato tubers. The difference in expression levels among different individuals reflects the variation in the insertion site of the transgene sequence in the plant genome and the copy number of the inserted gene. Considering the progress in vaccine and therapeutic agent production in transgenic plants, we hope that researchers will be able to overcome most of the issues in this field. Based on our research, we provide these suggestions for future studies (11, 15).
5.1. Conclusions
As the results obtained from this project mentioned above indicate, we have concluded that the Patatin promoter and TEV leader together can increase the expression of HBsAg in transgenic plants. Based on these findings, we offer the following suggestions for future studies.
- The expression cassette of HBsAg under the control of the Patatin promoter and TEV enhancer has been constructed.
- Three transgenic potato plants expressing HBsAg were grown and regenerated on selective media.
- The transgenic potatoes can produce a higher amount of HBsAg with the mentioned cassette compared to the cassette without the TEV leader.
- The TEV leader along with the Patatin promoter could have a synergistic effect for higher expression of the cloned gene compared to the TEV with the CaMV promoter.
ID number of HBsAg gene: 944569.