1. Background
A culture medium is essential for sustaining in-vitro-produced embryos longer than maturation or fertilization media (1). Therefore, accurately defining the substrate requirements for each developmental stage is crucial for optimizing the efficacy of embryo culture media (2). The culture medium comprises three main components: Serum, additive supplements, and growth factors. Serum is necessary in cell culture to enrich the medium, with fetal bovine serum (FBS) being the most commonly used in laboratories (3). Historically, FBS has been extensively used in vitro culture (IVC) media due to its inclusion of embryotrophic factors (4). Serum proteins serve multiple functions, including maintaining acid-base balance, osmotic pressure, pH stability, and providing amino acids (5). Fetal bovine serum contains essential components crucial for cell attachment, proliferation, and maintenance, such as serum albumin, fetuin, hormones, vitamins, trace elements, and growth factors, encompassing approximately 1800 proteins and over 4000 metabolites (6).
Despite these benefits, the precise effects of serum remain inconclusive (5). There are several drawbacks associated with FBS use, including its undefined composition and potential adverse effects (7). Notably, aberrant impacts on fetal growth, such as large offspring syndrome observed in cattle and sheep, have been linked to FBS supplementation in pre-implantation culture media (8). In contrast, chemically defined media offer precisely described components and concentrations (6), contributing to successful bovine zygote development to blastocysts (9). While defined media supplements are commercially available for certain cell types, their formulations often lack detailed disclosure (4).
Substituting FBS in cell culture media remains a significant challenge in cell and tissue culture (10). Current alternatives to FBS include components derived from human blood, such as plasma, serum, umbilical cord serum, and platelet derivatives like platelet lysate (PL) (11). Platelet lysate is derived from outdated human donor thrombocyte concentrates (12) and upon activation releases over 300 substances from intracellular granules (7), including adhesive proteins, coagulation factors, mitogens, protease inhibitors, proteoglycans (13), and a wide array of growth factors. Key mitogens found in PL include platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), transforming growth factor (TGF), and fibroblast growth factor 2 (FGF2), all exerting potent mitogenic effects. Additionally, PL contains cytokines and chemokines like interleukin (IL)-1β, IL-2, IL-6, IL-10, IL-12p70, IL-17A, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ (14). Platelet-derived growth factors, for instance, promote tissue repair mechanisms such as extracellular matrix remodeling, chemotaxis, cell proliferation, and differentiation (15).
Several culture media have been successful in supporting in vitro ovine zygote development to blastocysts, including Hams-F10, TCM199, Hams-F12, modified Brackets medium, synthetic oviductal fluid (SOF), Tyrodes medium, and various commercial media formulations. Charles Rosenkrans medium (CR1), for example, has been successfully used for bovine embryo culture (16). Platelet lysate has demonstrated efficacy in promoting proliferation across different cell lines, including tumor cells and articular chondrocytes (11), as well as in vivo expansion of various types of mesenchymal stromal cells (MSCs), endothelial colony-forming progenitor cells (ECFCs), and xeno-free propagation of human myoprogenitor cells (MPCs) (17).
2. Objectives
The current study aimed to examine the respective abilities of PL together with FBS in the modified Charles Rosenkrans medium (mCR2aa) compared to the BO-IVC medium in supporting the in vitro development of ovine embryos.
3. Methods
3.1. Location
All experiments were conducted in the Embryo Biotechnology Laboratory of the Iranian Research Organization for Science and Technology, maintained at a constant temperature of 27-31°C. Prior ultraviolet irradiation of all exposed surfaces was conducted during the night.
3.2. Chemicals and Media
All chemicals and media were sourced from Sigma-Aldrich (USA) and Gibco (USA), while plasticware was purchased from Falcon (USA). The commercial serum-free IVC systems, BO-IVM and BO-IVC, were obtained from IVF Bioscience (United Kingdom), and PL was procured from PL BioScience (Germany). All stock solutions and media were prepared using sterile triple-distilled Milli-Q water. Sterilization of the prepared media was achieved using membrane filters (0.22 µm).
3.3. Experimental Design
The aim of the present study was to reduce the percentage of FBS in the embryo culture medium and replace it with PL. Two experiments were designed for this purpose. The first experiment evaluated the effect of PL on in vitro embryo production by replacing FBS with PL in the mCR2aa culture medium. This experiment assessed concentrations of 0%, 2.5%, 5%, and 10% of FBS along with 0%, 2.5%, 5%, and 10% of PL. The second experiment compared the outcomes of the first experiment with those of the commercial BO-IVC medium. Parameters examined included fertilization rate, morula formation, blastocyst formation, and hatched blastocyst formation.
3.4. Oocyte Collection and In Vitro Maturation
Sheep ovaries were obtained from a regional abattoir and transported to the laboratory in 0.9% saline supplemented with penicillin (50 IU/mL) and streptomycin (50 μg/mL) within 2 hours of slaughter, maintaining a temperature of 35 - 37ºC in a flask container. Upon arrival, the ovaries underwent multiple washes in normal saline and were trimmed to remove excess tissue. Cumulus-oocyte complexes (COCs) were separated from follicles using the slicing method (18). Subsequently, the COCs were washed three times in a washing medium (TCM 199 medium containing sodium pyruvate, gentamicin, L-glutamine, and FBS). Morphological assessment of the COCs was conducted using a stereo zoom microscope, selecting only those with a compact, non-atretic cumulus oophorus, corona radiata, and homogeneous ooplasm for IVM. The oocytes were then cultured in groups of 15, placing the COCs into 100 μL droplets of BO-IVM in 5% CO2 in air for 24 hours at 38.5ºC (19, 20).
3.5. In Vitro Fertilization
For semen preparation, cryopreserved semen from a Romanov ram was rapidly thawed at 37ºC and washed twice using 10 mL of in vitro fertilization (IVF) medium (Brackett and Oliphant medium containing 10 mM caffeine sodium benzoate and 10 µg/mL heparin). The samples were washed twice by centrifugation at 700 g for 5 minutes at room temperature. The spermatozoa concentration was determined using a hemocytometer and adjusted to 1.0 × 107/mL through further dilution. A 50 µL aliquot of the sperm suspension was combined with a 50 µL droplet of IVF medium. Fifteen matured COCs were transferred to the 100 µL IVF medium droplet and incubated for 18 hours at 38.5ºC in a humidified atmosphere of 5% CO2 in air (21).
3.6. In Vitro Culture
After terminating fertilization, the putative zygotes were carefully pipetted to remove any remaining cumulus cells and adhering spermatozoa. Subsequently, the oocytes were washed several times using the IVC medium, which consisted of either BO-IVC or mCR2aa. The composition included 24.9 mM NaHCO3, 2.9 mM KCl, 108.3 mM NaCl, 2.5 mM calcium lactate, 1 mM glutamine, 0.5 mM sodium pyruvate, 0.5 mM glycine, 0.5 mM alanine, 1 mM glucose, 5 μg/mL phenol red, 50 μg/mL gentamicin, 2% Basal Medium Eagle amino acids, and 1% MEM non-essential amino acids, containing 0.6% bovine serum albumin and 10% FBS. Presumptive zygotes were randomly distributed into experimental groups. Embryonic development took place in a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 38.5ºC. The medium was replaced with 50% fresh culture medium every 48 hours. Cleavage rates were recorded on the second day following insemination. Additionally, development to the morula, blastocyst, and hatched blastocyst stages was recorded on the fifth, eighth, and ninth days following insemination (22).
3.7. Statistical Analysis
The data were expressed as mean ± Standard Error of the mean. A P-value less than 0.05 was considered statistically significant. Quantitative data analysis was performed using SPSS statistical software (version 16). Statistical analysis utilized the analysis of variance method followed by Tukey’s test.
4. Results
4.1. Effects of Optimization of Fetal Bovine Serum and Platelet Lysate Concentration on Modified Charles Rosenkrans Medium (mCR2aa) Culture Medium
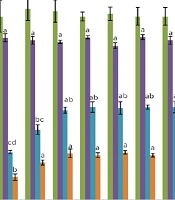
In this study, the effects of 0%, 2.5%, 5%, and 10% FBS along with 0%, 2.5%, 5%, and 10% PL in the mCR2aa culture medium were evaluated on the percentages of morula, blastocyst, and hatched blastocyst (Figure 1). The mCR2aa culture medium is a sequential medium, and a serum-free culture medium was used in the first two days for all treatments; therefore, no significant difference was observed in the zygote percentage among different treatments (P > 0.05).
Two days after embryo culture initiation, the use of different percentages of PL significantly reduced the percentages of morula and blastocyst compared to treatments containing serum (P < 0.05). When PL was used in the culture medium without serum, it caused the medium to gel.
Different concentrations of PL, combined with 2.5%, 5%, and 10% FBS, did not show significant differences in the percentage of morula. At a concentration of 2.5% FBS, the percentages of blastocysts and hatched blastocysts increased with increasing PL concentration; specifically, the percentages of blastocysts at concentrations of 5% and 10% PL were significantly higher than at 0%, and the percentages of hatched blastocysts at concentrations above 2.5% were significantly higher than at 0% (P < 0.05). However, at 0% FBS and 10% PL concentration, embryo adhesion to the bottom of the Petri dishes increased, causing handling problems.
When 5% FBS was used in the embryo culture medium, different concentrations of PL (0 - 10%) had no significant effect on the percentages of blastocysts and hatched blastocysts. At 10% FBS concentration, except for the 10% PL treatment, which significantly reduced the percentages of blastocysts and hatched blastocysts, no significant difference was observed between 2.5% and 5% concentrations compared to the control (10% FBS and 0% PL) (Figure 1).
4.2. Comparison of Modified Charles Rosenkrans Medium (mCR2aa), Optimized mCR2aa Medium, and BO-IVC Medium
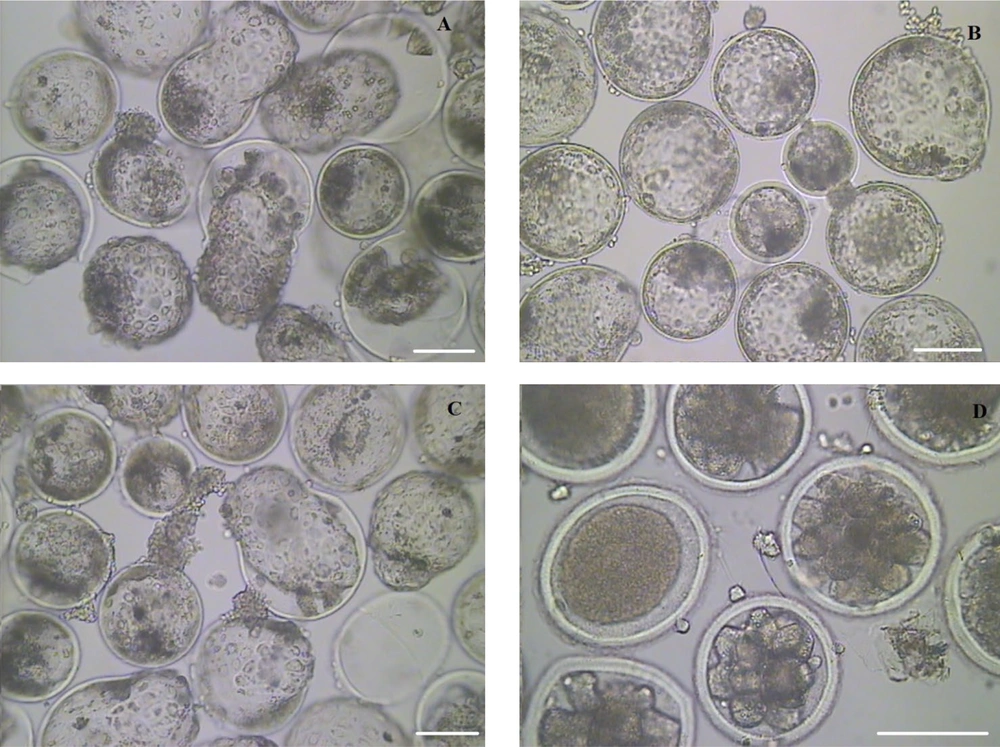
According to the findings of the first study, the performance of the mCR2aa embryo culture medium optimized with 2.5% FBS and 5% PL was investigated in comparison to the control mCR2aa containing 10% FBS and the BO-IVC commercial medium. No significant difference was observed in the percentages of zygotes, morulae, and blastocysts among the studied cultures. However, the percentage of hatched blastocysts produced was significantly higher in the BO-IVC medium compared to the other treatments (Figure 2). The embryos produced on the ninth day after fertilization in BO-IVC, mCR2aa (10% FBS and 0% PL), mCR2aa (2.5% FBS and 5% PL), and mCR2aa (0% FBS and 10% PL) media are shown in Figure 3.
Comparison of the Percentage of zygote, morula, blastocyst, and Hatched blastocyst in optimized modified Charles Rosenkrans medium (mCR2aa) treatments with 2.5% fetal bovine serum (FBS) and 5% platelet lysate (PL) with control mCR2aa containing 10% FBS and Bracket-Oliphant in vitro commercial culture medium [different letters indicate statistically significant differences (P ≤ 0.05)].
5. Discussion
The results of the current study indicate that using 5% PL in the mCR2aa culture medium allows for reducing serum percentage to 2.5%. The addition of serum can induce several changes in embryo morphology and metabolism, such as increased lipid droplets, enlarged blastocyst size, heightened apoptotic cell numbers, and altered mitochondria distribution (23). Researchers began exploring alternatives to animal serum over 30 years ago (24), leading to investigations and commercialization of potential substitutes for FBS. However, a cost-effective, universally applicable compound mimicking FBS composition remains elusive (25).
Platelets are noted for their abundance of growth factors, making them a promising alternative to FBS (26). Platelet lysates contain plasma proteins akin to those found in FBS (27) and have shown superior efficacy over recombinant growth factors in terms of cost and proliferation rates (28). Activated thrombocytes release potent mitogenic factors from α-granules, including PDGF, EGF, VEGF, bFGF, hepatocyte growth factor, and TGF-β1 (7). Supplements of PDGF at the various steps of in vitro production of bovine embryos (29), EGF in oocyte maturation (30), VEGF in porcine oocyte maturation (31), bFGF on maturation of mouse oocyte (32), HGF in ovine oocyte maturation (33) and TGF-b1 during inhibition of oocyte maturation in zebrafish have been used (34). Platelet lysate has been tested extensively as a serum substitute in human and animal cell lines (10), showing promising outcomes in ex vivo expansion and hematopoietic stem cell transplantation, surpassing serum in some aspects (7). However, studies investigating PL's effect on embryo culture medium as a serum substitute are limited.
Pazoki et al. demonstrated significantly increased maturation rates in media containing 5% PL or 5% PL + 5% FBS, while media with 10% PL or 10% FBS showed decreased maturation rates compared to control groups, underscoring the critical role of serum concentration in culture media (35). Furthermore, they observed a statistically significant increase in blastocyst production rates and total cell numbers in blastocysts cultured in media containing 5% platelet concentrate and 5% fetal calf serum (FCS) compared to controls and media with 10% platelet concentrate, highlighting the stimulatory effects of platelet-derived growth factors on bovine embryo development (36).
When PL was used in the culture medium without serum in this study, it induced a gel-like state in the culture medium. Additionally, at a concentration of 0% FBS and 10% PL, it led to increased embryo adhesion and posed handling challenges due to the absence of serum proteins. Serum proteins in embryo culture serve as a nitrogen source, chelate toxic metal ions, and act as antioxidants. Practically, without proteins in the culture medium, COCs and oocytes tend to adhere to plastic and glassware, making them difficult to manage (37).
The findings of this study revealed no significant difference in the percentage of morula and blastocyst between BO-IVC and mCR2aa culture media. However, the percentage of hatched blastocysts was significantly higher in the BO-IVC culture medium compared to the mCR2aa culture medium. Hajian et al. indicated that the main distinction between SOF and BO media was the blastocyst development rate, significantly higher in the BO medium than in the SOF medium. Nonetheless, there was no significant difference in developmental competence post-implantation between the two media. Messenger ribonucleic acid expression of evaluated genes in the SOF medium and somatic cell nuclear transfer embryos was higher than in the BO medium and IVF embryos, respectively. Furthermore, blastocyst formation rates were higher in the BO medium than in the SOF medium, suggesting that BO might be more suitable for IVC of caprine embryos (38).
5.1. Conclusions
The findings of this study indicated that PL alone could not fully replace serum. However, it is advisable to reduce the serum percentage to 2.5% and incorporate 5% PL in the embryo culture medium. Given the notable difference in the hatched blastocyst percentage between BO-IVC and the mCR2aa culture medium, it is recommended to further optimize the culture medium by including additional compounds, such as antioxidants necessary for embryo development.
![Effect of 0%, 2.5%, 5%, and 10% fetal bovine serum (FBS) with 0%, 2.5%, 5%, and 10% platelet lysate (PL) in Embryo Culture Medium on the percentage of Hatched zygote, morula, blastocyst, and hatched blastocyst [different letters indicate statistically significant differences (P ≤ 0.05)]. Effect of 0%, 2.5%, 5%, and 10% fetal bovine serum (FBS) with 0%, 2.5%, 5%, and 10% platelet lysate (PL) in Embryo Culture Medium on the percentage of Hatched zygote, morula, blastocyst, and hatched blastocyst [different letters indicate statistically significant differences (P ≤ 0.05)].](https://services.brieflands.com/cdn/serve/3170b/6e609736688c0623a3d740dac8c1fc1dc53b1c25/gct-146911-i001-F1-preview.webp)
![Comparison of the Percentage of zygote, morula, blastocyst, and Hatched blastocyst in optimized modified Charles Rosenkrans medium (mCR2aa) treatments with 2.5% fetal bovine serum (FBS) and 5% platelet lysate (PL) with control mCR2aa containing 10% FBS and Bracket-Oliphant in vitro commercial culture medium [different letters indicate statistically significant differences (P ≤ 0.05)]. Comparison of the Percentage of zygote, morula, blastocyst, and Hatched blastocyst in optimized modified Charles Rosenkrans medium (mCR2aa) treatments with 2.5% fetal bovine serum (FBS) and 5% platelet lysate (PL) with control mCR2aa containing 10% FBS and Bracket-Oliphant in vitro commercial culture medium [different letters indicate statistically significant differences (P ≤ 0.05)].](https://services.brieflands.com/cdn/serve/3170b/7fa18231a7a83fb0ef2b06e4b2a7af101655ed3a/gct-146911-i002-F2-preview.webp)