1. Background
5-hydroxytryptamine (5-HT) can induce changes in gastrointestinal motility and transmural transport of fluid and electrolytes (1-4). Although the 5-HT content of the gastrointestinal tract is higher than that of any other organ (5), the precise role of 5-HT in the physiology of the gut remains unknown. The difficulty encountered in establishing what endogenous 5-HT actually does in the gut may be due to the two different sites of 5-HT storage in the enterochromaffin cells of the mucosa (6) and in the enteric neurones, for which it serves a neurotransmitter role (7). Exogenous 5-HT exerts different actions in the gut through multiple receptor subtypes. For example, 5-HT1A receptors are present on the enteric neurons and ganglia (8), and presynaptically inhibit the release of acetylcholine at the nicotinic synapse and the secretion of tachykinins(9). 5-HT1A receptors are also located postsynaptically where they hyperpolarise the enteric neurons(10). 5-HT2 receptors are located directly on the smooth muscle, and the 5-HT3 receptors, which are ligand-gated ion channels are found in both submucosal and myentericneurones, which mediate a rapidly developing, but short lived postsynaptic depolarisation(11, 12). The 5-HT4 receptor, a neuronal receptor, which augments the release of acetylcholine at nicotinic synapses (10, 13), also increases the amplitude of contractions of the longitudinal muscle in response to transmural electrical stimulation in the guinea pig ileum(14). It isunknown whether endogenous 5-HT can access receptors available to stimulation by exogenous 5-HT to mediate the well-established contraction or relaxation response in the intestine.
2. Objectives
Therefore the aim of the present study was to electrically stimulate the rat intestinal segments in an attempt to release endogenous neurotransmitter substances. The possibility that released 5-HT may contribute to contraction and relaxation responses, was investigated using 5-HT receptor antagonists with selective actions for 5-HT receptor subtypes.
3. Materials and Methods
3. 1. Drugs
Atropine sulphate (Sigma), granisetron (GlaxoSmithKline), methysergide maleate (Sandoz), tetrodotoxin (Sigma), WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]- N- (2-pyridinyl) cyclohexanecarboxamidetrihydrochloride) (Research Biochemicals Inc), GR113808 ([1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate), SB269970-A (®)-3-(2-(2-(4-Methyl-piperidin-1-yl)ethyl)-pyrolidine-1-sulphonyl)-phenol, SB258585 (4-Iodo-N-[4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulphonamide) (Glaxo Smith Kline Beecham) and DL-PCPA methylester hydrochloride (Sigma) were dissolved in distilled water; ritanserin (Research Biochemicals Inc) was dissolved in methanol with distilled water being used for further dilutions. Preliminary experiments established that the vehicles used did not show any effect on the tissues or the responses to EFS.
3. 2. Preparation of Isolated Tissues
All procedures were carried out in accordance with institutional guidelines for animal care. Adult male Lister Hooded rats (Bradford University strain) (250-350 g) were killed by cervical dislocation following a blow to the head. The whole intestine was removed and immediately placed in freshly prepared Krebs’ solution (composition mM: NaCl 118, KCl 4. 7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2. 5, NaHCO3 25 and glucose 10) and gassed with 95% O2 and 5% CO2 at room temperature. The mesentery and fatty tissue were removed and the intestine was emptied of its contents by flushing Krebs’ solution gently through it using a narrow tipped pipette. Two segments were taken from four different regions of the intestine: duodenum, jejunum, mid ileum and terminal ileum, 1-5 cm, 10-14 cm, 30-34 cm distal to the pyloric region, and 1-5 cm proximal to the ileocaecal junction, respectively. Each segment was connected vertically to a tissue holder, which contained two stainless steel electrodes of 40 mm length, 80 mm apart.
After connecting the tissues to the tissue holders using cotton threads, the tissues were bathed in a 25 mL water-jacketed organ bath containing 20 mL Krebs’ solution and placed under a 1.0 g tension. The Krebs’ solution was maintained at a temperature of 37 ± 0.5oC and gassed continuously with a mixture of 95% O2 and 5% CO2. Each tissue was left to equilibrate in the presence or absence of the antagonist for 1 hour, and washed every 20 minutes. The resting tension was re-adjusted to 1.0 g when required throughout the experiment. Longitudinally mediated responses were recorded using isometric Grass transducers (FT03, Grass Instrument Co. , Mass, USA) and displayed, stored and analyzedusing a PC Pentium computer with the Power Lab Chart V4. 0.4 software.
3. 3. Method of Electrical Field Stimulation (EFS)
The effects of different drugs were observed in stimulated tissues. Stimulation of tissues was carried out using stainless steel electrodes extended from the Perspex tissue holders. Tissues were electrically stimulated using 200 BioScience stimulators. In all experiments double pulse stimulation with 75 ms delay between pulses and 0.5 ms pulse width at supramaximal voltage of 30 v and frequencies of 0.4, 1.0 and 10 Hz were used. The duration of the stimulation was 1 minute, which was applied at ten-minute intervals. Preliminary experiments revealed that these parameters were suitable to obtain reproducible responses for the entire duration of the experiments.
3. 4. Experimental Design
Using a paired experimental design, the frequency-response curves to EFS (0.4, 1.0 and 10.0 Hz) in the absence (control) or presence of antagonists were constructed. For the four selected regions of the intestine, one segment was randomly taken as the control and the others for tests. The test tissues were left to equilibrate with the antagonists atropine (muscarinic antagonist, 10 nM, 0.1 and 1.0 µM), WAY100635 (5-HT1A receptor antagonist, 1.0-10.0 µM(15)), methysergide (5-HT1/2/7 receptor antagonist, 1.0 µM), ritanserin (5-HT2 receptor antagonist 0.1 and 1.0 µM), granisetron (5-HT3 receptor antagonist, 1.0-10.0 µM), GR 113808 (5-HT4 receptor antagonist, 1.0 µM (16)), SB258585 (5-HT6 receptor antagonist, 1.0 µM (17)), SB269970A (5-HT7 receptor antagonist, 1.0 µM (18)) for one hour before the application of EFS. The antagonists were constantly present in the organ bath during the construction of the response curves. In separate experiments the effect of tetrodotoxin (TTX, 1.0 µM, 1 hour pretreatment) on the responses to EFS was studied. The number of observations ‘n’ represents the number of animals used. The profile of action of antagonists on responses to EFS was similar in different regions of the intestine; representative data is shown.
3. 5. Analysis of Results
Changes in g tension were expressed as either a percentage of the maximal response to KCl (0.1mM) or the mean of the absolute values plus standard error of the mean. The significance of differences between the control and the test responses was determined using the paired students’ t-test; values of less than 0.05 were considered statistically significant. The responses to the phasic and tonic components of the EFS applied at 10.0 Hz were measured during the first 10 seconds and 60 seconds of stimulation, respectively. The relaxation responses were analysed by measuring the minimum tonic response observed during the one-minute stimulation.
4. Results
4. 1. The Effect of EFS on Intestinal Tissues
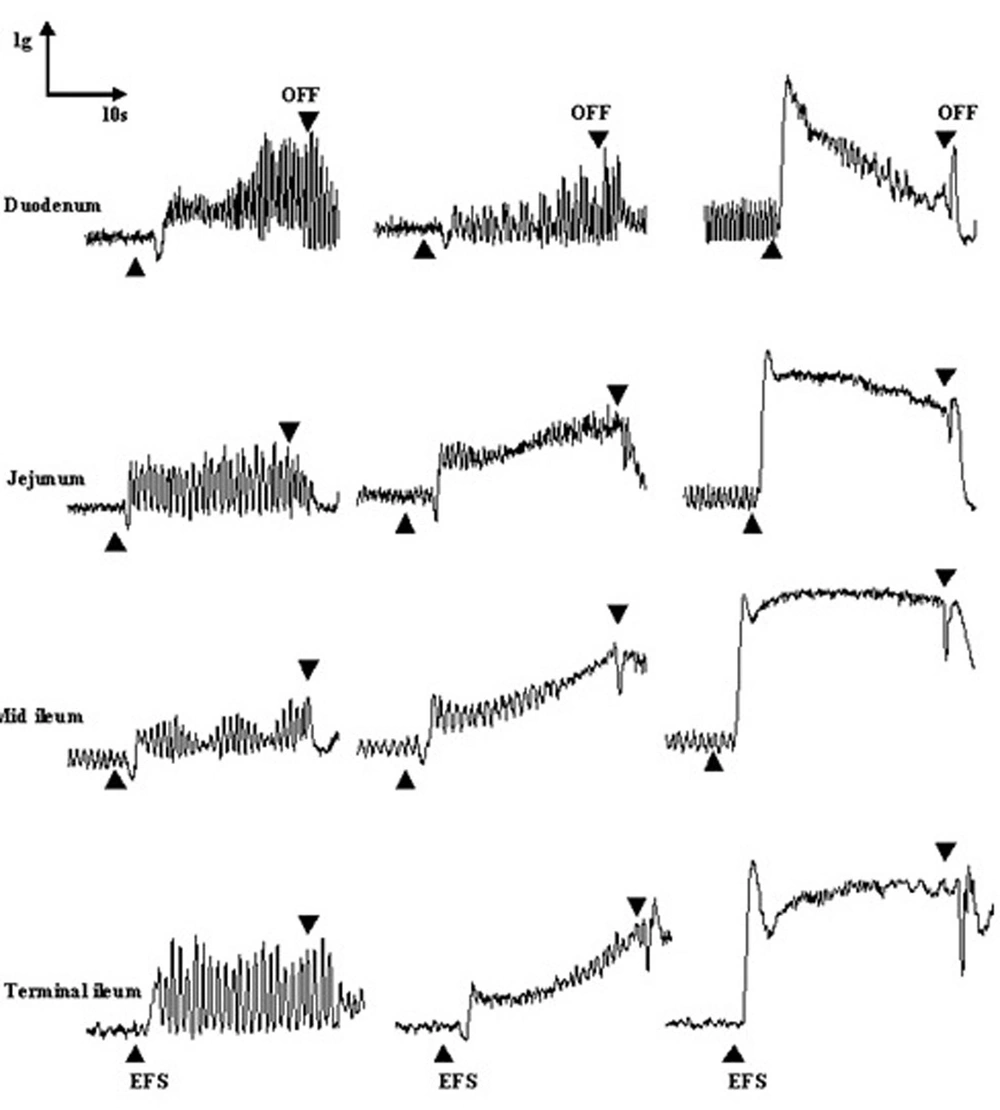
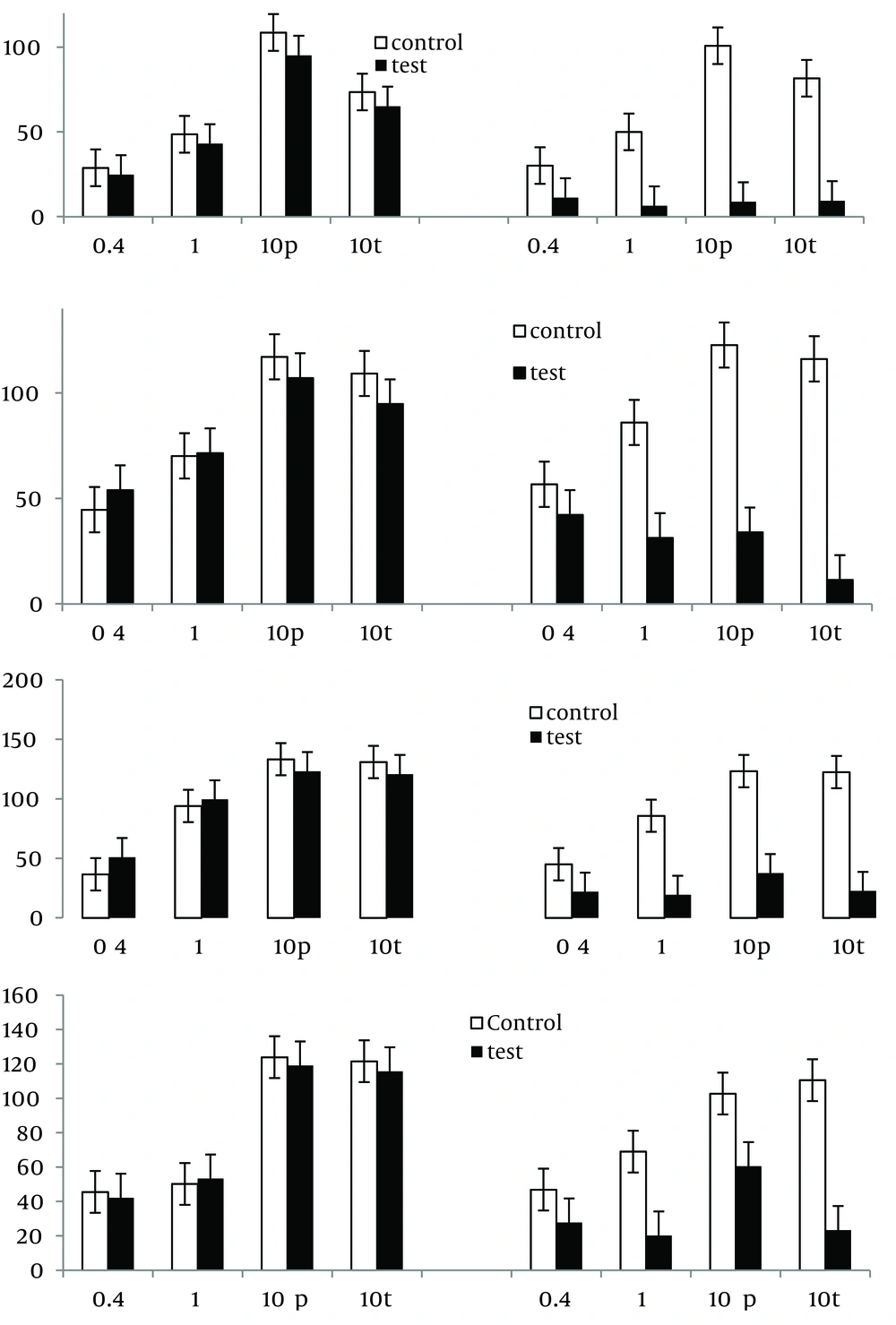
The application of EFS induced frequency-dependent contraction responses in all examined segments. The contraction response to EFS at low frequencies of 0.4 and 1.0 Hz developed slowly and proceeded by a small relaxation response in all examined tissues; EFS at a frequency of 10.0 Hz caused a rapid contraction (phasic) which was followed by a sustained contraction (tonic) (Figure 1).
4. 2. The Effect of Tetrodotoxin (TTX) on Modifying the Responses to EFS
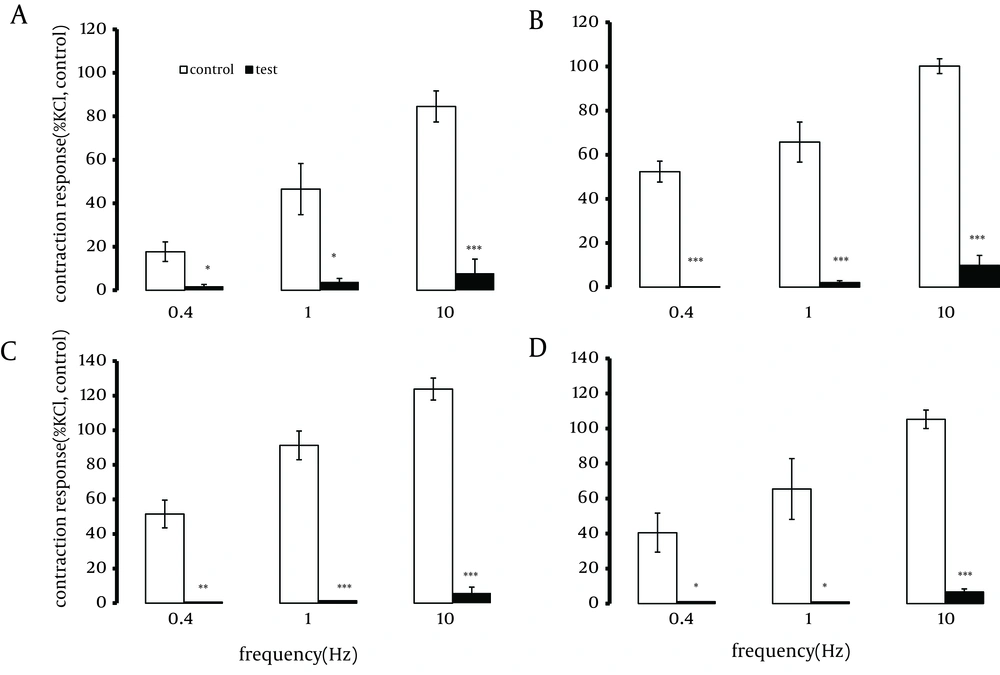
A one-hour pre-treatment with TTX ( 1.0 µM) significantly reduced (by approximately 90%) or abolished the contractions induced by EFS, at different frequencies (Figure 2).
Segments were taken from the duodenum (A), jejunum (B), mid ileum (C), and terminal ileum (D). Each point represents the mean ± SE. Total number was 6. *P < 0.05, **P < 0.01 and ***P < 0.001 indicate a significant difference compared to the control values. Contraction responses to EFS at 10Hz consist of two components of phasic (10 p) and tonic (10 t) contractions.
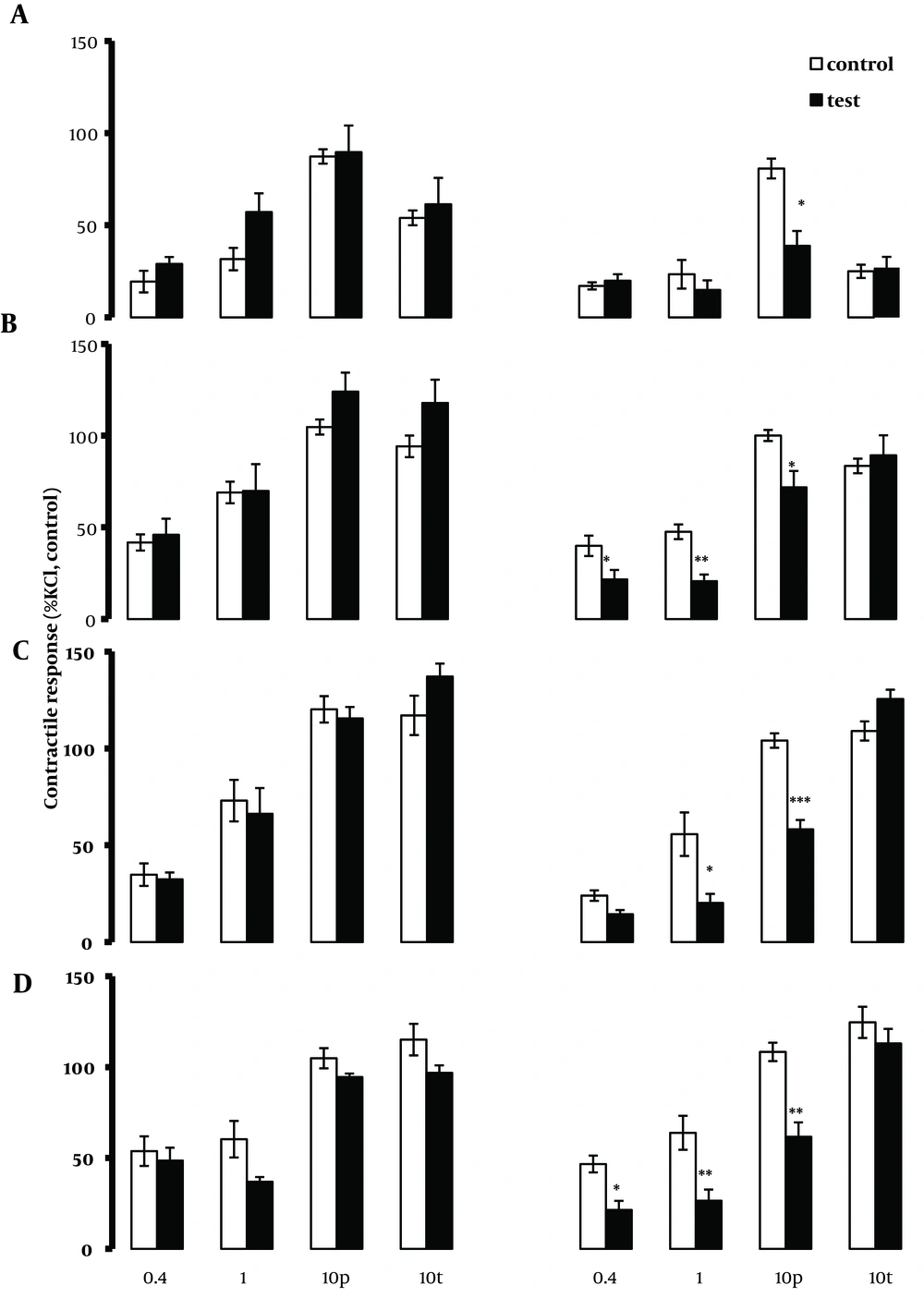
4. 3. The Ability of Atropine to Modify EFS Induced Responses
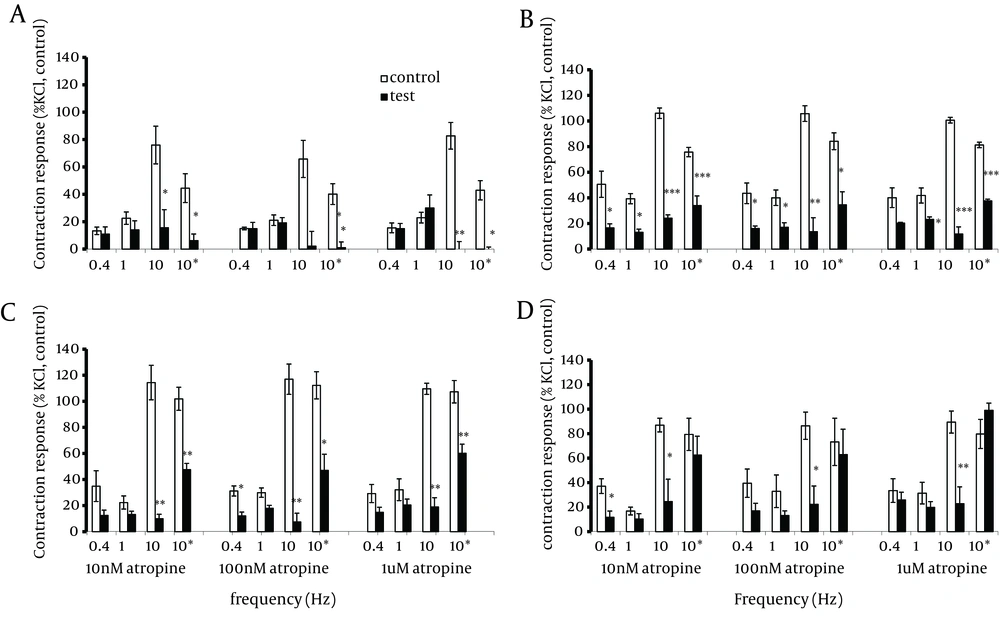
In tissues taken from the jejunum and ileum but not the duodenum, atropine (0.01, 0.1 and 1.0 µM) significantly (P < 0.05) reduced the contractions induced by EFS applied at low frequencies of 0.4 and 1.0 Hz. In the presence of atropine, the effect of EFS applied at 10.0 Hz was to induce a measurable relaxation response prior to the contraction response. Moreover, atropine significantly (P < 0.05-0.001) reduced or abolished the phasic contraction responses in all four regions of the intestinal tract (Figure 3). Furthermore, atropine was able to significantly (P < 0.05-0.001) reduce the tonic component of the contractile response to the EFS at 10.0 Hz in the duodenum, jejunum and mid ileum but not in the terminal ileum.
4. 4. The Ability of the 5-HT Receptor Antagonists to Modify EFS Induced Responses
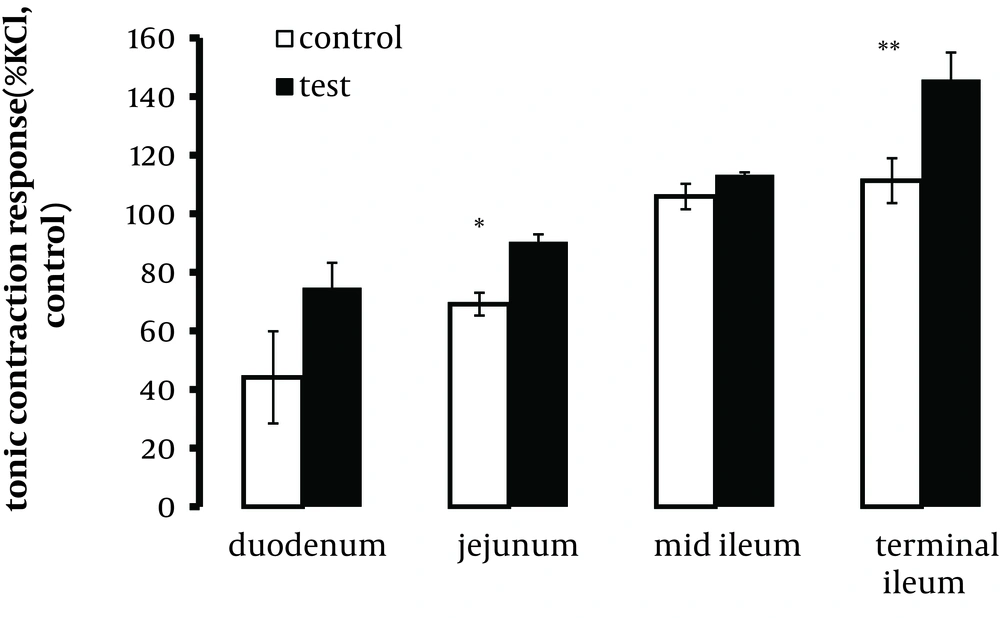
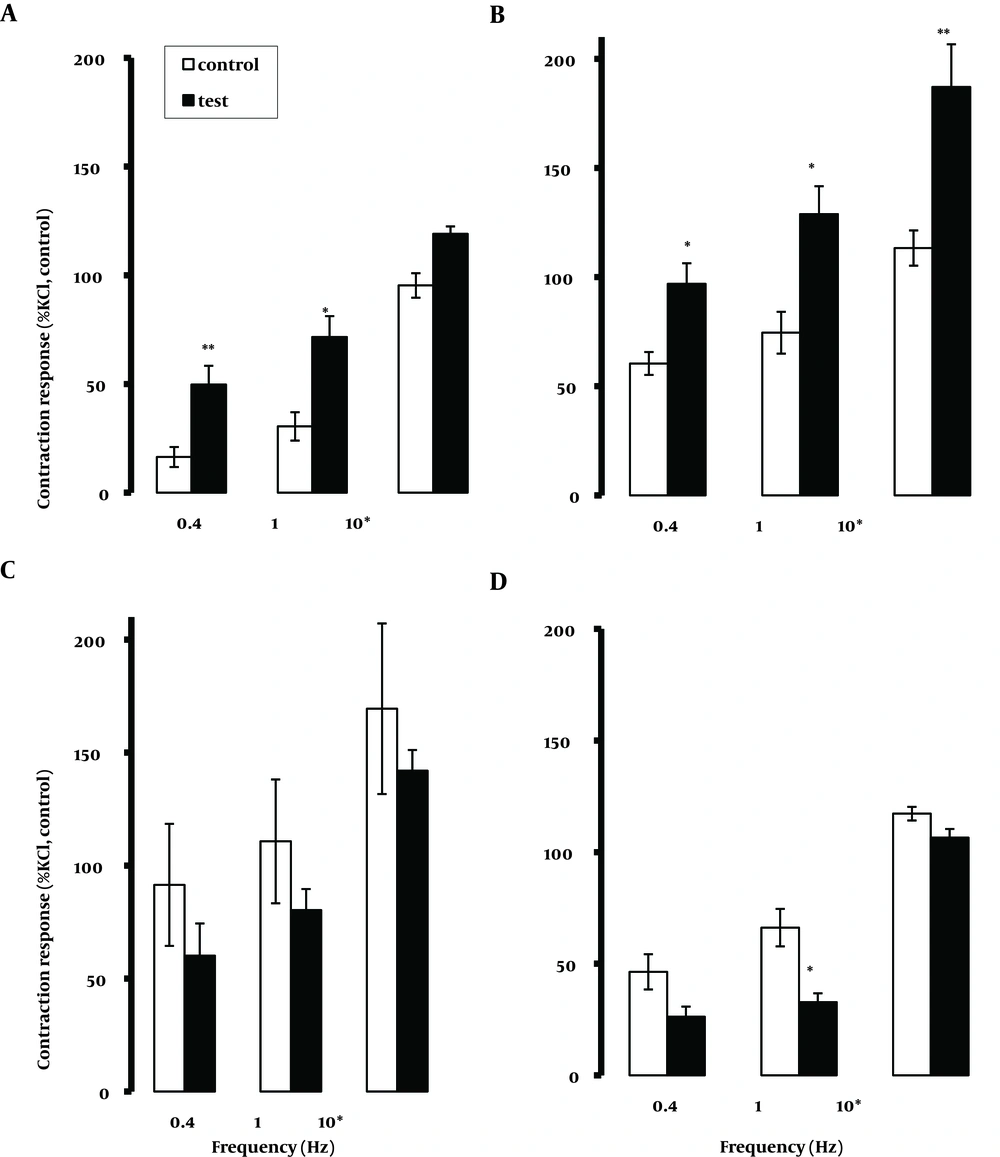
In the presence of the 5-HT1A receptor antagonist WAY100635 1.0, 10.0 nM and 1.0µM, the responses to EFS were comparable to those of control tissues in all segments examined. In most tissues examined, there was a trend for 10.0 µM WAY100635 to reduce the contraction response, which inconsistently achieved significance. However, the tonic contraction at 10.0 Hz was not reduced by 10.0 µM WAY100635 (data not shown). The relaxation response to EFS was not modified by WAY100635. Methysergide (0.1 and 1.0 µM) induced no changes in the contractile response to EFS, with the exception of a trend rising at a higher concentration of 1.0 µM to increase tonic contractions at 10.0 Hz that was significant in the jejunum and terminal ileum (P < 0.05 and P < 0.01, respectively), (Figure 4). The relaxation response to EFS reduced significantly in the duodenum in the presence of 1.0 µM methysergide.
The application of ritanserin at 1.0 µM but not at 0.01 and 0.1 µM, was able to significantly (P < 0.05) attenuate the contraction and the relaxation responses to EFS at all frequencies and in all tissues examined (Figure 5). In the presence of granisetron at 10.0 µM (but not at 1.0 µM) there was a general trend of attenuation of the contractile responses to EFS that frequently achieved significance (Figure 6). The application of GR113808 ( 1.0 µM) failed to modify the responses to EFS applied at different frequencies for all tested tissues (data not shown). In the presence of SB258585 ( 1.0 µM) the contractile response to EFS showed a general increasing trend in the proximal part of the intestine (duodenum and jejunum) that frequently achieved significance (P < 0.05, 0.01) (Figure 7). There was also a general trend to reduce the contractile response in the distal part (mid and terminal ileum). However it only achieved significance (P < 0.05) at 1.0 Hz in the terminal ileum. Pre-treatment with SB269970A ( 1.0 µM) induced an increase (P < 0.05) in the contractile response to EFS at 0.4 Hz in the segments taken from the duodenum. SB269970A failed to modify the contractile and relaxation responses to EFS when applied at other frequencies in any other region of the intestine (data not shown). Finally, in a separate in vivo experiment, pre-treatment of rats with para-chlorophenylalanine (PCPA), (250 mg/kg, i. p. , for 3 days) to deplete endogenous 5-HT (19), also failed to change EFS induced responses.
Segments were taken from the duodenum (A), jejunum (B), mid ileum (C), and terminal ileum (D. Each point represents mean ± SE. Total number was 5. *P <0.05 **P <0.01, ***P <0.001 indicate a significant difference compared to the control values; (10 p) and (10 t) represent phasic and tonic contractions.
Segments were taken from the duodenum (A), jejunum (B), mid ileum (C), and terminal ileum (D). Each point represents mean ± SE. Total number was 6. *P < 0.05, **P < 0.01, ***P < 0.001 indicate a significant difference compared to the control values; 10 p and 10 t refer to phasic and tonic contractions, respectively.
5. Discussion
The present study is the first systemic study performed on the rat small intestine from four different regions using all major serotonin receptor antagonists in an attempt to modify EFS induced contraction and relaxation responses. The principle aim of the present study was to investigate the possibility that endogenous 5-HT may contribute to the relaxation and contractile responses induced by electrical field stimulation in different regions of the rat small intestine. The systematic study of tissues gathered from throughout the length of the intestinal tract was considered important for revealing local differences in the functional importance of 5-HT. In such experiments, electrical field stimulation of tissues was used as an effective method for depolarizing nerves, and causing the release of neurotransmitters. A drawback to this technique is the fact that the stimulating current activates all neurons within the myenteric plexus, including excitatory and inhibitory elements, sympathetic and parasympathetic nerves and, cholinergic and non-cholinergic neurotransmitters (20). The muscle response to field stimulation is thus potentially the net result of multiple responses produced by activation of many individual neurons (21).
Different profile of muscle contraction and relaxation responses in different regions of the intestine might suggest that there are different endogenous neurotransmitters being released and/or interacting with different neurotransmitter receptors in different regions of the intestine. Furthermore, the release of neurotransmitters by the application of EFS at different frequencies might be predicted to modify the intensity or profile of the contraction-relaxation responses and this was found to occur in all tissues examined.
The EFS-induced contractions were markedly reduced or abolished by the sodium channel blocker tetrodotoxin (TTX) applied at a concentration that is shown to induce a maximal effect (22). This suggests that the existence of a major neuronal component is required to mediate EFS (0.4, 1.0 and 10.0Hz)-induced contraction in all four segments. Furthermore, at 10.0 Hz, when EFS induced only a very small relaxation or indeed no relaxation at all, the effect of TTX was to reveal a clear or enhanced relaxation in all tissues. Smooth muscle cell activation by substances released from enterchromaffin cells, for example, might thus contribute to the TTX-insensitive relaxation (23). In the present study, there was a consistent trend which sometimes achieved significance for contraction responses evoked by EFS at lower frequencies which reduced by atropine (10.0 or 100.0 nM) in tissues taken from the jejunum and ileum, indicating that a component of the contraction response is mediated via the release of acetylcholine. This profile of action was not observed in the duodenum indicating the involvement of other transmitters in the contraction response. The relaxation responses induced by EFS at lower frequencies were not consistently modified by atropine (10.0 nM to 1.0 µM). Indeed, in the presence of atropine, the effect of EFS applied at 10.0 Hz was to induce a measurable relaxation response prior to a contraction, which was normally absent in the control tissues. It can be concluded that the transmitter system for mediating relaxation response in the tissues is non-cholinergic.
It has been shown in other studies that nitric oxide and VIP contribute to the relaxation response induced by EFS in the longitudinal muscle of the mouse intestine (24) and rat gastric fundus (25) respectively, possibly involving a hyperpolarisation caused by an increase in potassium conductance. When EFS was applied at 10.0 Hz, atropine could abolish or greatly reduce the phasic contraction response in all regions and attenuate the tonic component of the contraction response in the duodenum, jejunum and mid ileum, but not in the terminal ileum. This indicates a difference in the cholinergic contribution to the responses induced by EFS in different regions of the intestine. A similar experiment on the guinea pig ileum (26) concluded that the initial phasic component was evoked by acethylcholine and by a non-cholinergic neurotransmitter, while the tonic component was maintained predominantly by prostaglandin released during stimulation. Ivancheva et al. (27) suggested that substance P could also contribute to the EFS induced tonic response.
In the present study the possibility that endogenous 5-HT may be involved in the atropine-insensitive residual contraction and relaxation responses induced by EFS, was first investigated using a 5-HT receptor antagonist with ‘selectivity’ for the 5-HT1A receptors, WAY100635 (pKB, 8. 7 in the rat brain (15, 28)). WAY100635, when applied in concentrations ranging from 1.0 nM to 1.0 µM, that have been shown in functional assays to block 5-HT1A-mediated effects at nanomolar concentrations (15), failed to block neither the contractions nor relaxations induced by EFS at low or high frequency stimulations. Even when applied at 10.0 µM, WAY100635 failed to consistently modify the EFS induced contractions and relaxations in the duodenum, jejunum and mid ileum. In the terminal ileum, a high concentration of WAY100635, reduced the contractions. However the mechanism of this inhibitory action of WAY100635 remains to be established. The ability of methysergide 1.0µM (a 5-HT1/2/7 receptor antagonist, pKi, 7. 1-8. 2 for 5-HT1/2receptors and pKi 7. 1-7. 9 for 5-HT7 receptors (29)) to increase the tonic contraction response to EFS when applied at 10.0 Hz only in the jejunum and terminal ileum, may indicate that the methysergide sensitive sites in these regions are involved in an inhibitory response. However, when methysergide was used in the presence of atropine, the tonic contraction was comparable to that of the control tissues, indicating that the inhibitory response mediated by methysergide-sensitive sites is cholinergic in nature (data not shown). This is in line with studies by Nowak et al. who showed evidence for the existence of muscarinic inhibitory neurotransmission in the rat small intestine upon the action of M1 muscarinic receptors located on inhibitory neurons (21). This may suggest a modulatory action by endogenous serotonin on cholinergic neurotransmission via methysergide sensitive sites.
The results of the present study indicate that the 5-HT2 antagonist, ritanserin (pKi 8. 5-7. 6 (30)), administered at nanomolar concentrations failed to modify relaxation and contractile responses to EFS. However at the higher concentration of 1.0 µM, ritanserin diminished EFS-induced contractions recorded from the rat intestine. It has been shown that the 5-HT2 receptors are involved in mediating a contraction response to the exogenously added 5-HT, following the administration of 10 nM – 0.1 µM ritanserin which could reliably block 5-HT2 receptors in theGIT of different species (31-33). Also, in in vitro binding assays in brain tissue (34), the antagonism by ritanserin was achieved at concentrations in the nanomolar range. This suggests that a reliable receptor blockage should be achieved with a 1.0 µM concentration of ritanserin. Thus, in the present study a strong antagonism of the EFS-induced contractions by 1.0 µM ritanserin might be attributed to the involvement of 5-HT2 receptors in mediating a response to endogenously released serotonin. However, such action of ritanserin at a concentration of 1.0 µM may also reflect additional non-specific action on other mechanisms (30) since ritancerin at 1µM was also able to reduce significantly the contractile response induced by KCl (100 mM).
It is known that agonists of the 5-HT receptors increase the release of acethylcholine from the motor nerve ending within the intestinal muscle and facilitate peristalsis and may be involved in mechanismsat 5-HT3 receptors (35-37). However, in the present study granisetron at a concentration as high as 1.0 µM failed to significantly modify relaxation and contractile responses to EFS. Although there was a significant reduction in contractile response to EFS in the presence of 10.0 µM granisetron, the selectivity of granisetron on 5-HT3 receptors at this concentration is questionable (38). Granisetron strongly and selectively binds to the 5-HT3 receptor with a binding constant of 0.26 nM and exhibits a 4000 – 40000 times greater binding affinity for the 5-HT3 receptor than other binding sites, including other 5-HT subtypes and also adrenergic, histaminergic and opioid receptors. Its selectivity to the 5-HT3 receptor over other receptor types is > 1000:1 (39). The lack of effect of the selective 5-HT4 receptor antagonist GR113808 on responses to EFS also indicates the unlikely involvement of 5-HT4 receptors. However, the existence of 5-HT4 receptors has been demonstrated in the gut (40). In the small intestine, 5-HT4 receptors mediate mucosal secretion and smooth muscle relaxation (41, 42). Furthermore, the 5-HT4 receptor has an established role in mediating contraction responses via a cholinergic mechanism in the guinea-pig intestine and colon (14, 43). In the present study, the lack of evidence for the involvement of 5-HT4 receptors in mediating a response to EFS could be that the receptors do not play a dominant role in mediating a response to endogenously-released 5-HT under normal physiological conditions. There is also evidence that the enterochromaffin cells are endowed with 5-HT4autoreceptors and that their stimulation causes inhibition of 5-HT release (44).
In the present study, pre-treatment with SB258585, a selective 5-HT6 receptor antagonist with high specific binding (17) at a concentration of 1µM, induced different effects depending on the frequency of EFS used and the region of the intestine; a significantly greater contraction response to EFS at 0.4 and 1.0 Hz in the duodenum and jejunum, and a reduction of EFS-induced contraction at the same frequencies in the ileum was observed. Application of EFS at higher frequency of 10.0 Hz induced a greater contractile response for both the tonic and phasic components of contraction only in segments taken from the jejunum. Furthermore, a greater relaxation response was observed in the presence of antagonist in some intestinal segments. To date there is no functional evidence for the involvement of 5-HT6 receptors in the periphery. Thus the present study could be the first evidence for the involvement of 5-HT6 receptors in EFS-induced response upon endogenously released serotonin, which require further investigations.
The application of the selective 5-HT7 receptor antagonist SB269970A at a concentration of 1µM induced a greater contractile response to EFS at low frequency of 0.4 Hz only in segments taken from the duodenum. This indicates the involvement of 5-HT7 receptors in a relaxation response to the endogenous 5-HT as antagonism of these receptors resulted in a greater response. This is in line with previous studies where the 5-HT7 receptor was implicated in a relaxation response in the gastrointestinal tract (45-47). In summary, the application of 5-HT receptor antagonists, revealed that methysergide- (5-HT1/2/7 receptor), ritanserin- (5-HT2 receptor), SB258585- (5-HT6 receptor) and SB269970- (5-HT7 receptor) sensitive sites might be involved in the endogenous 5-HT mediating contraction or relaxation response to EFS. However, considering the concentration of antagonists applied for the present study, and the fact that at concentrations lower than 1µM, none of antagonists were able to consistently modify the EFS induced contractile or relaxation responses. Even, in case of depletion of 5-HT from enteric neurons by PCPA failed to modify EFS response, may suggest the unlikelihood of direct involvement of endogenous 5-HT in mediating contraction or relaxation responses to EFS in different regions of the rat small intestine. Accordingly, recent studies have shown that endogenous serotonin is neither required for colonic peristalsis in vitro, nor gastrointestinal (GI) transit in vivo (48, 49).