1. Background
Chronic periodontitis (CP) is an infectious inflammatory disease characterized by the destruction of tooth-supporting structures, wherethe presence of specific microorganisms are required, but not sufficient for disease development (1). Cytokine networks play an important role in the initiation and progression of periodontal disease. Pro-inflammatory cytokines play crucial roles in microbe-induced destructive inflammation (2). During the progression of periodontal inflammation, periodontal ligament and gingival fibroblasts secrete high levels of cytokines and chemokines (3). Production of numerous pro-inflammatory cytokines is amplified by several bacteria-derived virulence factors, thereby leading to the destruction of soft tissues and bones (4). Tumor necrosis factor-alpha (TNF-α) is a cytokine that has been implicated in periodontal disease due to its effects on bone and soft tissue metabolism (4). It is an endotoxin induced macrophage hormone, which is implicated in the pathogenesis of shock and cachexia (5). It has a high potential for increasing bone resorption and is involved in the degradation of connective tissue by stimulating prostaglandin-E2 and Collagenase, respectively (4). Several studies have reported elevated levels of TNF-α in both GCF and gingival tissue biopsies from periodontal disease sites of humans. Furthermore, blockage of the activity of TNF-α was found to slow down the progression of experimental periodontitis in primates (6-8).
TNF-α is known to be the most important pro-inflammatory cytokine released at the site of periodontal disease and is considered as the most potent osteoclast activating factor (OAF) (9). A number of studies have shown a close association between TNF-α level and periodontal disease severity and also some studies showed that, during active periodontitis, TNF-α served as a marker of tissue destruction, bone resorption (10-13), and clinical severity(7). TNF-α inhibits collagen synthesis and at high concentrations stimulates collagenase synthesis in fibroblasts (14).Polymorphisms in genes that encode immunological molecules such as pro-inflammatory cytokines have been targeted as potential genetic markers for periodontitis. These cytokines play an important role in the initiation and amplification of the inflammatory response (15, 16).The biological basis for the association between cytokine gene polymorphism and periodontal disease is that carriage of certain alleles is suspected to cause changes in the production of a given mediator (12). Numerous studies suggest that the severity of periodontitis may differ in carriers of rare alleles of single cytokines, such as the 308A allele of tumor necrosis factor-α (TNF-α) gene (10, 17-19). Polymorphisms in the promoter region of the TNF-α gene at position (308, G to A) have been evaluated (13, 20, 21). The 308 A allele has been associated with high level of promoter activity, and enhanced TNF-α production (10, 22). Galbraith et al. (23) found that patients with periodontal disease carrying the rare allele at position 308 of the TNF-α gene had higher TNF-α production than the non-carriers. More studies are required in order to verify the association of TNF-α gene polymorphisms with the level of tissue destruction and periodontal disease progression. To date, there has been no data concerning the stereological parameters of gingival tissues of individuals with TNF-α gene polymorphisms.
2. Objectives
The present study investigated the stereological parameters of interdental gingiva in CP patients with TNF-α (-308 G/A) gene polymorphisms.
3. Materials and Methods
3.1. Sample Selection
The patients were selected according to the criteria specified in the international workshop for classification of periodontal diseases and conditions (24). The patientswere non-smokers and none of them had a history or current manifestation of systemic diseases. Patients with severe medical disorders such as diabetes mellitus, immunological disorders, hepatitis and pregnant womenwere excluded from the study. After approval ofthe University Ethics Committee (No. 89-2880) and obtaining written informed consent from the participants, 2 mL peripheral venous blood were collected in Na-EDTA tubes from each subject to detect TNF-α (-308 G/A) polymorphisms.
3.2. DNA Extraction and Genotyping
DNA was extracted from whole blood by salting out method as described previously (16) . A Tetra primer amplification refractory mutation system–polymerase chain reaction (TARMS-PCR) was designed to detect TNF-α (-308 G/A) polymorphisms (25).
3.3. Preparation of Tissues and Stereological Study
Tissue preparation was done,according to the protocol described (3), as follows: After determination of (TNF-α) (-308 G/A) gene polymorphisms using T-ARMS-PCR, 45 interdental gingiva tissues (40 GG, 4 GA+1 AA genotype) were considered as the case group. Because of only one sample with AA genotype, the GA and AA genotypes were considered as a group and were compared with the GG genotype group. The gingiva in the control group (n=15) were obtained during tooth extraction for orthodontic or prosthodontic treatments from the persons with clinically healthy gingiva. The mean age in the chronic periodontitis group was 42.2 ± 8.93, and in the control was 39.5 ± 8.19 years. The interdental papilla tissues were immersed in Lillie’s fixative solution for one week at room temperature. After tissue processing and embedding in paraffin wax, each interdental gingiva sample was exhaustively sectioned into 4μm thick sections. Ten to 13 sections were sampled from each specimen by systematic uniform random sampling (SURS) (3, 26). The first section of tissue was randomly obtained between 1 to 200micron and the next sections with a 200micron distance were selected by SURS and stained with Masson’s trichrome. Cavalier’s point counting method was employed to estimate the volume of interdental papilla (3). On each sampled section six to eight fields were selected in a SURS manner. A test system of points was then superimposed on these fields and points hitting the various components of the tissue were counted. Then an estimate of the volume density (Vv) of the components in the reference space was obtained using:
Vv = P (part) /P (ref)
Where, P (part) and P (ref) are respectively the number of test points falling in all structure profiles and in the reference space (3, 27). All stereological analyses were done by two expert histologists on blind-coded slides.
3.4. Statistical analysis
Data were presented as means ± SD and 95% confidence interval (95% CI). Student t test was used to compare differences between groups. Significant level was P < 0.05. All statistical analyses were performed employing SPSS version 16.0 for Windows software system.
4. Results
Basic and clinical parameters of sampling site in CP patients and healthy controls were described previously. Histological structure of C and CP patients are shown in Figure 1 and Figure 2. Quantitative analysis of gingival sample indicatedthat there were statistically significant differences in the volume density (Vv) of epithelium, connective tissue, collagenous and non-collagenous matrix, and blood vessels between control and all chronic periodontitis groups (P < 0.0001) (Table 1). There were no statistically significant differences in the Vv of epithelium and connective tissue of gingiva, collagenous and non-collagenous matrix and blood vessels between GG, and GA+AA groups (P > 0.05) (Table 2).
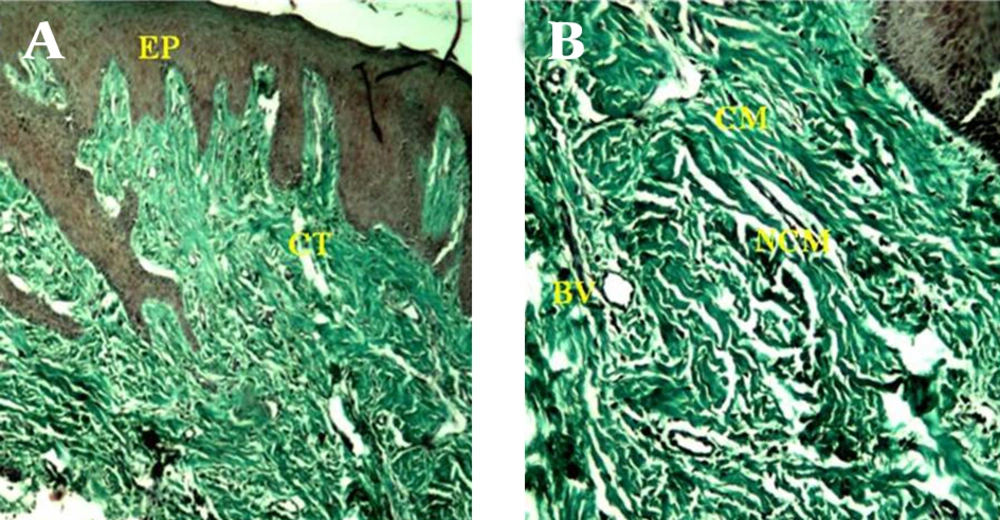
A) Consists of a surface epithelium (EP) and supporting connective tissue (CT) (40 X). B) Gingival connective tissue in the control sample consists of collagenous matrix (CM) , and non-collagenous compartment that in turn comprises non-collagenous matrix (NCM) and blood vessels (BV) (100 X).
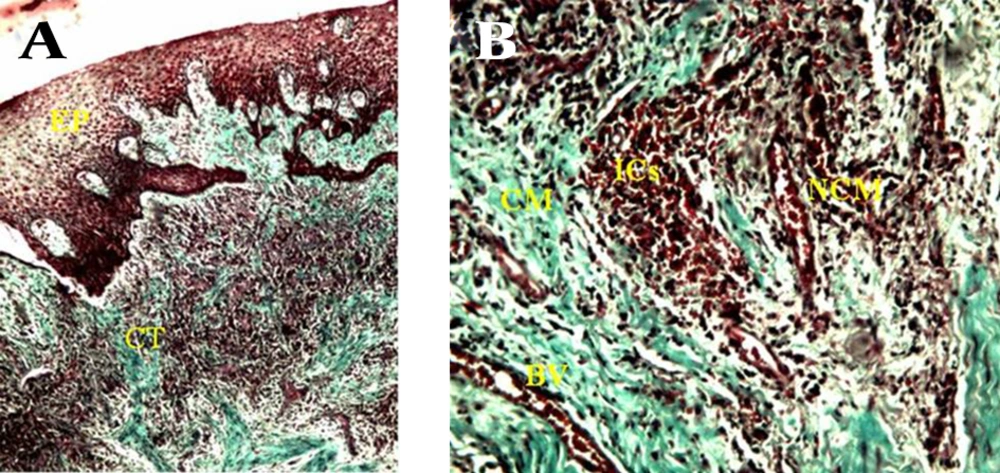
A) consists of a surface epithelium (EP) and supporting connective tissue (CT) (40 X). B) Gingival connective tissue consists of collagenous matrix (CM) and non-collagenous compartment that in turn comprises non-collagenous matrix (NCM) and blood vessels (BV) , note degradation of collagenous extracellular matrix (CM) associated with an infiltration of several inflammatory cell populations in non-collagenous matrix (NCM) ,increment of blood vessels (BV) and infiltration of inflammatory cells (ICs) (100 X).
| Volume density | CP (n = 45) | Control (n = 15) | P Value c | ||
|---|---|---|---|---|---|
| Results, mean ± SD | 95% CI | Results, mean ± SD | 95% CI | ||
| Epithelium | 36.7 ± 4.32 | 35.44-38.03 | 27.8 ± 3.73 | 25.74-29.86 | <0.0001 |
| Connective tissue | 63.3 ± 4.32 | 62.00-64.56 | 72.2 ± 3.73 | 70.14-74.26 | <0.0001 |
| Collagenous matrix | 35.4 ± 7.14 | 33.21-37.50 | 58.3 ± 3.35 | 56.48-60.19 | <0.0001 |
| Non-collagenous compartment | 27.7 ± 6.11 | 25.90-29.57 | 15.6 ± 3.37 | 13.39-16.75 | <0.0001 |
| Blood vessel | 16.3 ± 4.56 | 14.90-17.64 | 8.8 ± 1.82 | 7.79-9.81 | <0.0001 |
| Non-collagenous matrix | 11.7 ± 5.48 | 10.02-13.35 | 6.6 ± 2.59 | 5.13-7.94 | <0.0001 |
a Abbreviations: CI, confidence interval; CP, chronic periodontitis
b Data are presented in percentage.
cP < 0.0001 compared to the control group
| Volume density | GG Genotype (n = 40) | GA + AA Genotype (n = 15) | P Value c | ||
|---|---|---|---|---|---|
| Results, mean ± SD | 95% CI | Results, mean ± SD | 95% CI | ||
| Epithelium | 36.6 ± 4.42 | 35.18-38.02 | 37.8 ± 3.56 | 33.38-42.22 | NS |
| Connective tissue | 63.4 ± 4.42 | 61.98-64.82 | 62.2 ± 3.56 | 57.78-66.62 | NS |
| Collagenous matrix | 35.5 ± 7.42 | 33.10-37.9 | 34.4 ± 4.72 | 28.54-40.26 | NS |
| Non-collagenous compartment | 27.7 ± 6.31 | 25.71-29.74 | 27.8 ± 4.66 | 22.02-33.58 | NS |
| Blood vessel | 16.2 ± 4.74 | 14.71-17.74 | 16.6 ± 3.21 | 12.62-20.58 | NS |
| Non-collagenous matrix | 11.8 ± 5.68 | 9.93-13.57 | 11.2 ± 2.77 | 7.75-14.65 | NS |
a Abbreviation: NS, not significant.
b Data are presented in percentage.
cOne way ANOVA
5. Discussion
Results of the current study indicated that there were statistically significant differences in quantitative parameters of interdental gingiva between the CP patients and the healthy controls. It is well known that in periodontal diseases there is local infiltration of inflammatory cells associated with a degradation of extracellular matrix (ECM) macromolecules. Collagen fibers quantitatively constitute the major component of the ECM of gingival connective tissue, and have a primordial role in its architecture. Loss of the collagen component may reflect the severity of the CP (3, 28-30). Results of the current study showed that there was a statistically significant difference in the Vv of gingival epithelium and connective tissue between the CP patients and the healthy controls. The gingival epithelium is the most external tissue compartment mainly implicated in the defense of the deeper periodontal tissues, and it is an important physical barrier against external pathogens. Increment of Vv of epithelium might be due to hyperplasia and infiltration of inflammatory cells in epithelium (3). Several studies have suggested a substantial genetic influence in CP. Host modifying factors associated with severe periodontitis suggest a biological mechanism by which some individuals, if challenged by bacterial accumulations, may have a more vigorous immune-inflammatory response, leading to more severe clinical diseases(31).
Among the host proteases that target the ECM, the matrix metalloproteinases (MMPs) have been especially associated with the remodeling of periodontal tissues. MMPs are usually found in balance with a group of endogenous proteins named tissue inhibitors of metalloproteinases (TIMPs), to keep matrix remodeling highly regulated. In fact, MMPs and TIMPs are regularly expressed in healthy periodontal tissues, where they are supposed to control the ECM physiological turnover(1). While the presence of periodontal pathogens is required, but not sufficient, for disease onset, studies have clearly demonstrated that the host response plays a critical role in periodontal tissue breakdown (32). TNF-α acts in the cell migration process at multiple levels, inducing the up-regulation of adhesion molecules and the production of chemokines, which are chemotactic cytokines involved in cell migration to infected and inflamed sites. Supporting the data from human studies, analysis of data for experimental periodontal disease in rats and primates clearly demonstrated that TNF-α plays a central role in inflammatory reaction, alveolar bone resorption, and the loss of connective tissue attachment (1). In addition to presenting a direct effect on the pathogenesis of PD, TNF-α up-regulates the production of other classic pro-inflammatory innate immunity cytokines, such as IL-1β and IL-6. Interestingly, IL-1β and IL-6 have been characteristically associated with inflammatory cell migration and osteoclastogenesis processes (1, 32). Several genetic, environmental, ethnical, and sexual factors as well as the type of periodontal bacteria can affect the disease process. There are strong evidences indicating greater impact of genetic factors than environmental ones on periodontitis development (25). Polymorphisms in genes encoding molecules of the host defense system, such as cytokines, have been targeted as potential genetic markers. It is conceivable that individual differences in periodontitis susceptibility or individual differences in CP severity are related to genetically determined differences in TNF-α production and secretion by a variety of cells. It has been shown that carriers of the TNF-α 308 A allele appeared to have greater transcription activity, and produced higher levels of TNF-α (33). Moreover there wasa higher prevalence of allele A TNF-α-308 in periodontitis patients compared to those suffering from gingivitis (23). TNF-α (-308 A > G) gene polymorphism affects the expression of this cytokine. In addition, the presence of A allele in this region wasaccompanied by increased level of TNF-α and intensified periodontal disease (34, 35). On the other hand, de Jong et al. suggested that TNF gene polymorphisms were not related to differences in levels of endotoxin induced TNF production in whole blood samples (36). The authors’ previous study also indicated that there was no association between TNF-α (-308G > A) polymorphism and chronic periodontitis in the population (25). Results of the present study showed that there was no statistically significant difference in the Vv of epithelium, connective tissue, collagenous matrix and non-collagenous compartment of gingival connective tissue and blood vessels between GG, and GA+ AA polymorphisms of TNF-α (-308 G/A) gene polymorphisms in CP patients. One reason for these results may be population heterogeneity. Disease prevalence pattern often changes with geography and ethnic origin, and allele frequencies can vary widely worldwide. In other words, a different allele of a single SNP may be a marker for certain phenotype in different population samples. A genetic risk factor for disease susceptibility in one population may not be a risk factor in another population sample (16). The limitation of this study was its relatively small sample size. Consequently, subgroup analysis was not possible. Larger studies are needed to confirm these findings on the relationships of genetic variations to the pathogenesis of CP. However, more comprehensive studies in larger groups of patients on genotype and allele diversity of TNF-α gene polymorphism, and molecular mechanisms by which TNF-α is involved in susceptibility to CP are necessary.