1. Background
Tuberculosis (TB) caused by Mycobacterium tuberculosis, remains a major health problem. WHO predicted about 8.6 million cases of tuberculosis and 1.3 million death from the disease In 2012 (1). The incidence rate of TB in Zahedan (central of Sistan and Baluchistan province) is higher than other regions of the country (2). Tuberculosis predominately affects the lungs, but can affect almost all of the body organs, including the brain, the kidneys and the bones. Only 5% to 10% of the human beings who infected with mycobacterium develop the active disease in their life span and 90% remain as latent. Host genetic variation, environmental and characteristics of the mycobacterium strain play important roles in susceptibility to TB. Toll-like receptors (TLRs) are a transmembrane protein playing key role in the innate immune system. TLRs are the first line of protection against pathogens and have a critical role in inflammation and immune regulation (3). They are usually expressed on various immune cells such as macrophages and dendritic cells, which recognize certain molecules derived from bacteria (4). They belong to a receptor superfamily with Interleukin-1 receptors recognized as Interleukin-1 Receptor / Toll-Like Receptor Superfamily. Indeed, they are a family of pattern-recognition receptors (PRR) and recognize pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), lipoproteins and peptidoglycans (5). Stimulation of TLRs by PAMPs leading to the activation of NF-κB transcription factor which induces the secretion of several proinflammatory cytokines, production of interferon (IFNs) lead to activation of macrophage in response to mycobacterium (6). Thirteen TLRs have been characterized in humans, each one recognizes different PAMPs from various microbial pathogens, such as viruses and bacteria. Some of these TLRs are localized on the cell surface such as TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11, and the rest are located on endosomal/lysosomal compartment such as TLR3, TLR7, TLR8, and TLR9 (7). It seems that TLR1, TLR2, TLR4, TLR6, TLR8 and TLR9 are involved in the recognition of mycobacterium (8).
Toll-like receptor 8 is encoded by the TLR8 gene also known as CD288. It is located on the Xp22.2 chromosome close to another family member, TLR7 (9). TLR8 is expressed in monocytes, macrophages and myeloid dendritic cells (DCs) and is able to recognize single-stranded RNA and short double-stranded RNA of pathogens (10). Toll-like receptor 9 is encoded by the TLR9 gene, which is also known as CD289. It is located on 3p21.3 chromosome (11). TLR9 is expressed in different cells of the immune system such as dendritic cells, B lymphocytes, monocytes and natural killer (NK) cells, and is able to recognize unmethylated CpG islands in DNA molecules leading to production of cytokines such as type-I interferon and IL-12 (12). TLRs activation by its ligands cause a lot of biological processes such as cytokine secretion, apoptosis and antimicrobial activity (8). Several studies investigated the role of TLRs polymorphism in patients with pulmonary tuberculosis (PTB), but the results of genetic association studies are controversial.
2. Objectives
Therefore, the aim of the present study was to investigate the association between TLR 8, 9 polymorphism and PTB patients and healthy individuals.
3. Patients and Methods
This case control study was performed in the Research Center for Infectious Diseases and Tropical Medicine, Bou-Ali Hospital, Zahedan, Iran. A total of 160 patients with pulmonary tuberculosis (newly diagnosed PTB cases and underwent treatment subjects for PTB), and 160 unrelated healthy subjects with no clinical symptoms or family histories of TB belonged to same ethnicity as patients and living in the same region as the patients with PTB (Southeast Iran) were enrolled in the study. The Ethics Committee of Zahedan University of Medical Sciences approved the project, and informed consent was obtained from both PTB patients and healthy control subjects. Tuberculosis was diagnosed by clinical symptoms, radiological evidence, sputum Acid Fast Bacillus (AFB) smear positivity, culture and response to antituberculosis chemotherapy as described in our previous study (13). Two milliliters of venous blood was collected from each patient and healthy subject using EDTA as anticoagulant and stored at -20 °C until further use. Genomic DNA was isolated from peripheral blood samples using salting out method as described previously (14). We searched the TLR8, 9 genes in the SNP database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/SNP,) and selected two SNPs: rs3764880 A/G among the TLR8 and rs148805533 among the TLR 9 SNPs.
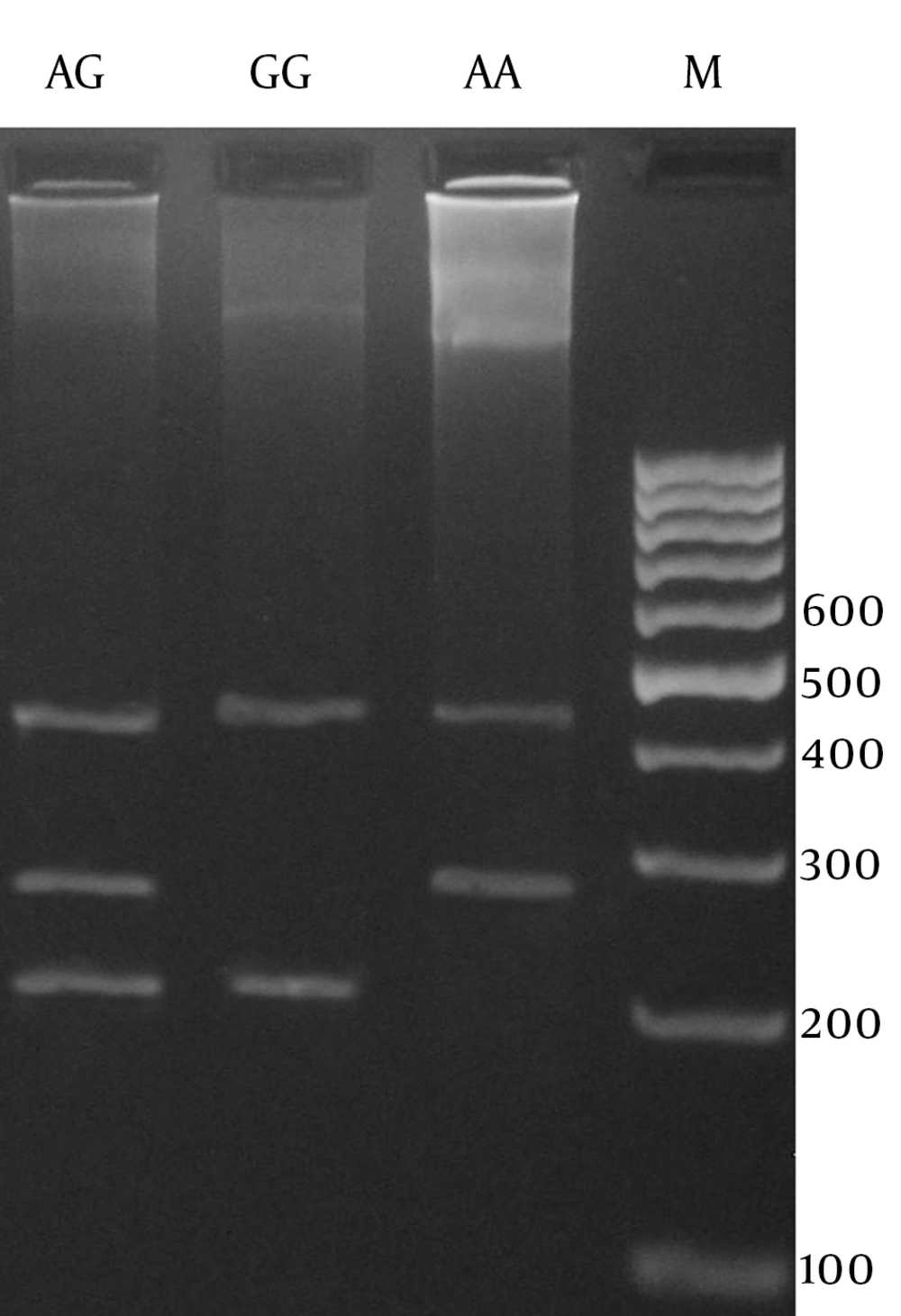
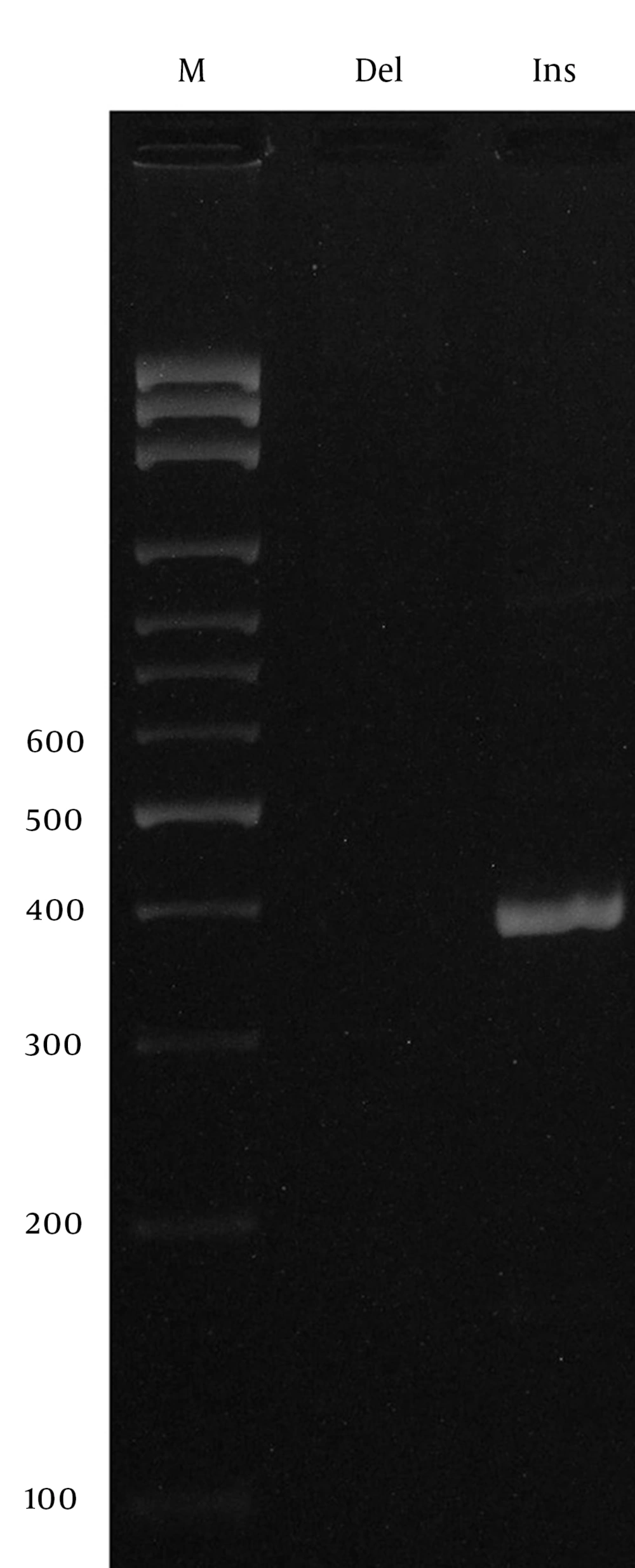
In this study, we designed a Tetra amplification refractory mutation system polymerase chain reaction (T-ARMS PCR) for detection of TLR8 polymorphism, and allele specific PCR for detection of TLR9 polymorphism. We used four primers in T-ARMS PCR for detection of TLR8 SNP rs3764880 A/G, two external primers (forward outer: 5’- AAATCACAAGTTCCCTTCTTTTCATGTA -3’, reverse outer: 5’- CATCACTGCATTTGATTTTCAAAATTTA -3’) and two internal primers (forward inner [A allele]: 5’- GGAATGAAAAATTAGAACAACAGAACCA 3’ reverse inner [A allele]: 5’- TTTGCTAAAGAAATAGAAGTGGCTTACAAC -3’). Primers for the TLR9 rs148805533 polymorphism were as follows: Forward (Del allele): CAGGGCCATGTCACTGTTGC, Forward (Ins Allele): CAGGGCCATGTGGGTGACAA, Reverse: GTAGGCTGTCTGAGAGGGGA. The PCR mixture included 0.4 μM of each primer, 250 μM of each dNTP, 1 U Taq DNA polymerase with 2 mM MgCl2, and 50 ng genomic DNA. Polymerase chain reactions (PCRs) were performed as follows: 5 min at 95°C followed by 30 cycles of 30 s at 95°C, 30 s at 50°C for rs3764880, 30 s at 64°C for rs148805533, and 30 s at 72°C, and final extension was performed at 72°C for 5 min. PCR products were analyzed by electrophoresis on 2% agarose gel and observed under ultraviolet light.
The product sizes of rs3764880 polymorphism were 272bp for A allele, 209bp for G allele and 423bp for the two outer primers (Figure 1). The PCR products for insertion and deletion alleles (rs148805533) comprised 411bp and 397 bp, respectively (Figure 2). Random samples were regenotyped to verify the genotyping accuracy. Regenotyping confirmed the previous results and there were no genotyping errors. Statistical analysis was performed by using SPSS for Windows, V18.0, SPSS, Inc., IL, USA. To investigate potential association of the selected polymorphisms and tuberculosis, the allele and genotype frequencies in patients and healthy controls were compared using Pearson’s chi-squared test. Logistic regression analysis was applied to estimate odds ratio (OR) and 95% confidence intervals (CI) of genetic risk in PTB. A P value less than 0.05 was considered as statistically significant.
4. Results
The study subjects included 160 PTB patients (mean age ± SD: 51.27 ± 20.52) and 160 healthy controls (mean age ± SD: 47.68 ± 15.86 years). Among the PTB patients, 77 were males and 83 were females, and among the healthy controls, 62 were males and 98 were females. There was no significant difference among the PTB patients and healthy controls regarding sex and age (P > 0.05). Table 1 summarized the genotype and allele frequencies of rs3764880 A/G polymorphisms in females. There were no significant differences between female patients with pulmonary TB and female control ones for genotype frequencies regarding the TLR8 rs3764880 polymorphism. The wild-type genotype AA was observed in 43 (43.9%) of the female patients, whereas 34 (34.7%) were heterozygote (AG) and 21 (21.4) were homozygote for the mutant genotype (GG). In the female control group, the frequencies of genotypes were 40 (48.2%) for AA and 16 (19.3%) for AG and 27 (32.5%) for GG. No significant differences were found in allelic frequencies between PTB and control subjects females regarding rs3764880 polymorphism of TLR8 (P = 0.51). As shown in Table 2 similarly, there were no significant differences between the male pulmonary TB and male control cases for genotype distribution frequencies at rs3764880 (p > 0.05). The analysis of the PTB patients and healthy control revealed no statistically significant difference between the groups regarding 14-bp insertion/deletion polymorphism of the TLR9 gene. All of the samples had Ins/Ins genotypes. Our finding demonstrated that the 14-bp deletion polymorphism of TLR9 is not a risk factor for PTB.
| Polymorphism | PTB | Normal | OR (95% CI) | P Value |
|---|---|---|---|---|
| Codominant | ||||
| AA | 43 (43.9) | 40 (48.2) | 1.00 | - |
| AG | 34 (34.7) | 16 (19.3) | 1.97 (0.0949-4.11) | 0.06 |
| GG | 21 (21.4) | 27 (32.5) | 0.72 (0.35-1.47) | 0.37 |
| Dominant | ||||
| AA | 43 (43.9) | 40 (48.2) | 1.00 | - |
| AG + GG | 55 (46.1) | 43 (51.8) | 1.2 (0.66-2.10) | 0.56 |
| Recessive | ||||
| AA + AG | 77 (88.6) | 56 (67.5) | 1.00 | - |
| GG | 21 (21.4) | 27 (32.5) | 0.56 (0.29-1.10) | 0.09 |
| Alleles | ||||
| A | 120 (61.2) | 96 (57.8) | 1.00 | - |
| G | 76 (38.8) | 70 (42.2) | 1.15 (0.75-1.75) | 0.51 |
a Abbreviation: PTB, pulmonary tuberculosis
b Data are presented as No. (%)
| Alleles | PTB | Normal | OR (95% CI) | P Value |
|---|---|---|---|---|
| A | 48 (62.3) | 42 (67.7) | 1.00 | - |
| G | 29 (37.7) | 20 (32.3) | 1.15 (0.84-1.59) | 0.80 |
a Abbreviation: PTB, pulmonary tuberculosis
5. Discussion
The objective of the current study was to detect the presence of rs3764880 polymorphism in TLR8 and rs148805533 polymorphism in TLR9 genes and to assess its distribution among patients with tuberculosis, and healthy subjects in a sample of Iranian population. Our findings revealed that there were no significant differences between female pulmonary TB and female control groups for genotype frequencies regarding TLR8 rs3764880 polymorphism; similar to females, there were not significant differences between the male pulmonary TB and male control cases. In agreement with our findings, Dalgic et al. reported that genotype distributions and allele frequencies at rs3764880 between the PTB and control groups did not show significant differences. They did not find any significant differences between the female pulmonary TB and female control groups for genotypes GG, AG and AA, or between alleles G and A frequencies at rs3764880. However They found a significant association between genotype A/(−) and susceptibility to PTB in males (15). In contrast to our findings, Davila et al. in a study on PTB patients from Indonesia and Russia found that four polymorphisms in the TLR8 gene were associated with TB susceptibility in males, that one of them was polymorphism rs3764880, In addition they found a strong allelic association with susceptibility to PTB in males (9). They demonstrated that the mechanism through TLR8 recognizes mycobacterium and intracellular signaling is unknown, however they mentioned that expression level of TLR8 were up-regulated significantly in patients during the acute phase of disease (9). TLRs have a critical role in pathogen recognition and activation of innate immunity and act in multiple cellular processes such as cytokine secretion (6), modulation of the adaptive immune response and apoptosis (8). TLR8 is located on X chromosome and is activated by bacterial nucleic acids (10). TLR8 has a role in immunity against mycobacterium through IRF-7 and induced production of IFN(10).
No significant difference was found between PTB patients and healthy control regarding rs148805533 polymorphism in TLR9 genes in our population. In agreement with our study Selvaraj et al. in south India showed that allele and genotype frequencies of TLR9 were similar in healthy control subjects and patients with pulmonary tuberculosis, and polymorphism in TLR9 is not associated with susceptibility to pulmonary TB in south Indian population (12). Jahantigh et al. in a study on TB-endemic region of Iran (Zahedan) showed that genotype and allele frequency of -1486 T/C TLR9 were not significantly different between pulmonary TB patients and controls (16). Contrary with our results Velez et al. showed that five SNP in TLR9 had statistically significant associations with TB and concluded that TLR9 might play important roles in determining susceptibility to TB (11). Several prior studies also found that variants of TLR9 are associated with TB susceptibility (17, 18), furthermore the role of TLR9 polymorphisms in other infectious diseases, inflammation and cancers have verified by many researchers (19-22). TLR9 is expressed intracellularly by different cells of the immune system and has a key role in activation of the innate immune system against mycobacterium infection (17). TLR9 alerts the immune system by binding to unmethylated CpG DNA motifs and induction of IL-12 production through DCs (8).
Polymorphisms within TLR9 may impair the immune response against TB, and it is thought that polymorphism in TLR9 may influence their expression, function and affecting RNA splicing (8, 17). In conclusion, our results indicated that TLR8 rs3764880 polymorphism and TLR9 rs148805533 polymorphism may not be major genetic factors for susceptibility to pulmonary tuberculosis in a sample of Iranian population. Additional studies in a large number of TB patients and functional studies are needed to validate our findings.