1. Background
Uterine leiomyoma (UL) or fibroid is a noncancerous tumor originating from the myometrium (1). ULs are the most common genital neoplasms in women and may be the most prevalent tumors in in their reproductive age (2, 3). The prevalence of UL is about 30% to 40% in women in reproductive age; it becomes symptomatic in near one-third of patients and is the most common indication for hysterectomy (4). Although the related pathophysiology and proliferative pathway of this disorder is not clear yet, several studies suggested that the genetic, hormonal, anthropometrical, and reproductive factors might play important roles in developing UL (5, 6). The epidemiological studies suggested that early menarche is associated with increasing risk of fibroids (7, 8). Similar to ovarian and breast cancer, ULs are estrogen dependent as estrogen induces cell proliferation in different tissues including uterus (9, 10). Some evidences suggested that cytokines might have a key role in development of ULs. Elevated levels of some cytokines such as interleukin 4 (IL-4) have been observed in uterine cavity of patients with UL in comparison with the normal uterus (11, 12). The human IL-4, which is mapped on the long arm of chromosome 5 (5q31- 33), is produced by CD4+ helper T cells (Th2 cells), basophils, and mast cells (13). IL-4 has cytotoxic and anti-tumor effects that inhibits induction of nitric oxide synthase and therefore, inhibits release of superoxide by macrophages (14). There are several specific single nucleotide polymorphisms (SNPs) and variable number of tandem repeats (VNTRs) in cytokine genes that could play major roles in genetic predisposition to some diseases and cancers. Recent investigations have revealed that some of these polymorphisms could alter the cytokines production levels (15). There is a 70-base-pair (bp) VNTR polymorphism in the third intron of the IL4 gene that may alter the expression level of this gene. Three alleles for the IL4 gene VNTR polymorphism have been reported: RP*1 allele, three repeats; RP*2 allele, two repeats; and RP*3 allele, four repeats. The RP*1 allele is more frequent than RP*2 allele and RP*3 allele is the rarest one, which has been observed in few populations (16). Investigations showed that RP*3 allele is associated with high production of IL-4 (17). There is only one published report concerning the association of IL4 gene VNTR polymorphism with UL development (18).
2. Objectives
In the present study, we aimed to assess the probable association between 70-bp VNTR polymorphism of the IL4 gene and the risk of UL in southeast of Iran.
3. Patients and Methods
3.1. Patients and Sample Collection
This case-control study included 99 premenopausal women with clinically diagnosed UL who had underwent myomectomy or hysterectomy in Ali-Ebne-Abitaleb Hospital, Zahedan, southeast Iran, between 2012 and 2013. UL was diagnosed by detailed ultrasound examination and was confirmed by histopathological examination after myomectomy or hysterectomy. The control group consisted of 102 premenopausal women who were referred for routine yearly check-up and performing the Pap smear test; the controls were matched with the patients for age, ethnicity, and body mass index (BMI). The absence of UL was determined in controls after detailed ultrasound examination. All samples collection were approved by Ethics Committee of Zahedan University of Medical Sciences.
3.2. DNA Analysis
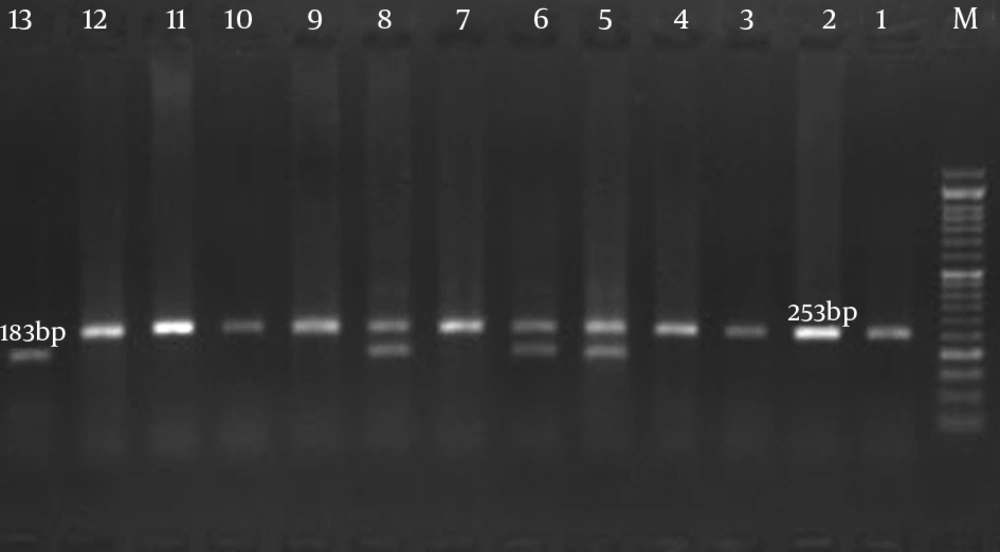
The DNA analysis was performed in the Cellular and Molecular Research Center (Zahedan, Iran). The DNA was extracted from whole blood using the salting-out method. The 70-bp VNTR region of IL4 gene was analyzed by polymerase chain reaction (PCR) using two oligonucleotide primers: forward, 5'AGGCTGAAAGGGGGAAAGC-3′; and reverse, 5′CTGTTCACCTCAACTGCTCC-3 ′ (19). PCR was performed on a 25-μL final volume contained 25 pmol of each primer, 0.1 mmol of dNTP (Fermentas, Lithuania), 0.5 μg of genomic DNA, 1.5 mmol of MgCl, 2.5 μL of PCR buffer, and 1.5 unit of Taq DNA polymerase (Fermentas, Lithuania); the process was performed according to the following protocol: initial denaturation at 94℃ for five minutes; 30 cycles of denaturation at 94℃ for 50 seconds, annealing at 61℃ for 30 seconds; extension at 72℃ for 45 seconds; and final extension at 72℃ for five minutes. PCR products were separated by electrophoresis on a 1.5% agarose gel and were visualized by ethidium bromide staining (Figure 1).
3.3. Statistical Analysis
All statistical analyses were performed using the SPSS v.15.0 (SPSS Inc, Chicago IL, USA). Direct gene counting method was used to determine the allele frequency. The independent effect of the IL4 VNTR genotypes on UL was examined by logistic regression analysis. The odds ratio (OR) and 95% confidence intervals (CI) were also estimated. The χ2 test was used for deviation of genotype distribution from Hardy-Weinberg equilibrium. In all tests, P value less than 0.05 was considered statistically significant.
4. Results
The demographic data of patients with UL and control group are shown in Table 1. There were no significant differences in age, BMI, and marital status between two groups. No significant differences in menstrual history including age at menarche, duration of menses, and duration of the menstrual cycle were found between the control and patients. The frequency of bleeding and pain were significantly higher in patients than in controls (P < 0.0001). Deviation from the Hardy-Weinberg equilibrium for IL4 VNTR polymorphism was observed in neither patients nor controls (P > 0.05). The allelic and genotypic frequencies of IL4 VNTR polymorphism in patients and controls are shown in Table 2.
The frequency of RP*1/RP*1, RP*1/RP*2, and RP*2/RP*2 genotypes were respectively 70%, 24%, and 6% in patients with UL and respectively 80%, 19%, and 1% in controls. There were no significant differences in frequency of RP*1/RP*2 versus RP*1/RP*1 genotype between patients and controls, even after adjustment for age. Although the frequency of RP*2/RP*2 was not significantly higher than RP*1/RP*1 genotype in patients in comparison with the controls (OR, 2.7; 95% CI, 0.9-7.8; and P = 0.07), increased risk of UL was observed in individuals with RP*2/RP*2 genotype after adjustment for age (OR, 4; 95% CI, 1.3-12.4; and P = 0.015). The frequency of PR*2 allele were 18% and 10% in patients and healthy controls, respectively, which showed a significant difference (OR, 1.9; 95% CI, 1.1-3.5; P = 0.03).
| Patients (n = 99) | Controls (n = 102) | P Value | |
|---|---|---|---|
| Age, y | 38.5 ± 9.8 | 36.7 ± 5.5 | NS |
| Marriage status | NS | ||
| Married | 98 | 100 | NS |
| Unmarried | 1 | 2 | NS |
| BMI, Kg/m2 | 25.3 ± 4.8 | 25.4 ± 4.7 | NS |
| Age at menarche, y | 13.1 ± 1.5 | 13.5 ± 1.1 | NS |
| Duration of menses, d | 6.6 ± 2.7 | 5.8 ± 1.5 | NS |
| Menstrual cycle, d | 28.6 ± 2.3 | 29.1 ± 3.4 | NS |
| Bleeding | 57 (58) | 6 (6) | 0.0001 |
| Pain | 28 (28) | 8 (8) | 0.0001 |
5. Discussion
In this investigation we focused on the association of IL4 VNTR polymorphism with UL development in southeast of Iran. There was not any association between RP*1/RP*2 genotype of IL4 VNTR polymorphism and UL development even after adjustment for age. Although the frequency of RP*2/RP*2 genotype was not statistically higher than RP*1/RP*1 genotype in patients in comparison with the controls, the frequency was significantly higher after adjustment for age.
UL is the most prevalent noncancerous tumor in women. Although 75 out of 100 women have UL, only around 25% of patients present with its symptoms and signs (2). Several studies have suggested that UL arises from a single neoplastic smooth muscle of myometrium (20). Moreover, evidences have shown the association between serum levels of cytokines and UL development (18). Some studies showed that several cytokine genes polymorphism are associated with different cancers such as breast cancer (21), cervical cancer (22, 23), gastric cancer (23), hepatocellular carcinoma (24), and ovarian cancer (25, 26).
These observations supported the hypothesis that immunological, inflammatory, and anti-inflammatory processes could play key roles in tumor development (12). A few studies reviewed the association of IL4 SNPs with UL development and some of the studies indicated the association between these SNPs and UL (27). Recently, the association of IL4 VNTR polymorphism with several diseases such as cervical cancer (19), breast cancer (28), end-stage renal disease (29), systemic lupus erythematosus (30), oral cancer (31), preeclamsia (32) and multiple sclerosis (33) have been investigated. Shekari et al. showed that the frequency of RP*1/RP*2 genotype of IL4 VNTR polymorphism was significantly higher in patients with cervical cancer than healthy women, which is similar to our results (19). The correlation between IL1beta-511 promoter polymorphism and increased frequency of UL has been reported by Pietrowski et al. and Taghizade-Mortezaee et al. (34, 35). Moreover, Litovkin et al. showed a trend for association between IL6 -174C allele and developing UL (36). Sosna et al. reported that CC genotype of IL4 -590 C > T and -33 C > T polymorphisms were less frequent in patients with UL in comparison to the controls (27).
There is only one published report concerning the association between IL4 VNTR polymorphism and UL by Hsieh et al. in 2007 (18). Although they found a slight increase in the frequency of RP*1/RP*2 and RP*2/RP*2 genotypes in patients with UL in comparison with women without UL in Taiwan, the differences were not statistically significant, which is consistent with our results before adjustment for age. Moreover, the frequency of RP*2 allele in patients with UL was not significantly different from women without UL, which was similar to our results. Our study suffered from some limitations. Firstly, our sample size was small that could affect the results. Secondly, if we could perform the study on both myomatous and normal tissues, the results would be more valuable. To promote the knowledge concerning the role of IL4 VNTR polymorphism in UL pathogenesis, more extensive studies with larger numbers of people including other ethnic groups from the viewpoint of genetic and environmental factors should be performed.
In conclusion, there was an association between IL4 VNTR RP*2/RP*2 genotype and developing UL after adjustment for age; therefore, this genotype could be an age-related risk factor for UL. Moreover, the frequency of RP*2 allele was significantly higher in women with UL.