1. Background
The immune response to different types of cancer has been the subject of many previous researches. Over years, several aspects of this topic have been considered, including the relative lymphocytosis in the peripheral blood and the histological presence of immunoreactive cells around and inside the neoplastic tissue or in the metastatic regional lymph nodes. These studies have been addressed also to lung neoplasia, mainly focused on non-small-cell lung cancer (NSCLC), demonstrating that a local infiltration by immunocompetent cells, primarily lymphocytes, correlates to a better prognosis (1-6).
2. Objectives
We investigated 20 patients’ immune response to small-cell lung cancer (SCLC), in order to evaluate the state around and inside the neoplastic tissue. For this reason, particular attention was paid to observe the presence of tumor-infiltrating lymphocytes and to recognize neo-lymphangiogenesis. The presence of a connective stromal network was also carefully evaluated.
3. Materials and Methods
Nowadays, surgical resection specimens of SCLC are absolutely rare, therefore our investigation was performed on bronchopulmonary biopsies. In our series we included 20 cases with classic SCLC, not combined with other histological types of lung cancer. None of the patients had received treatments prior to their surgeries. There were 18 male and two female patients, aged 56-74 years in the study group, without signs of immunological diseases and with no previous or concomitant steroid therapy. All bioptic samples with an evident of necrotic background were excluded. The diagnosis was based on the typical SCLC morphology, with the standard hematoxylin-eosin staining and all ascertained by immunohistochemistry for CD56 and chromogranin. The implementation of a new immunostaining method with the D2-40 antibody, specifically against podoplanin (7), permitted the evaluation of both lymphatic capillaries and the neo-lymphangiogenesis.
4. Results
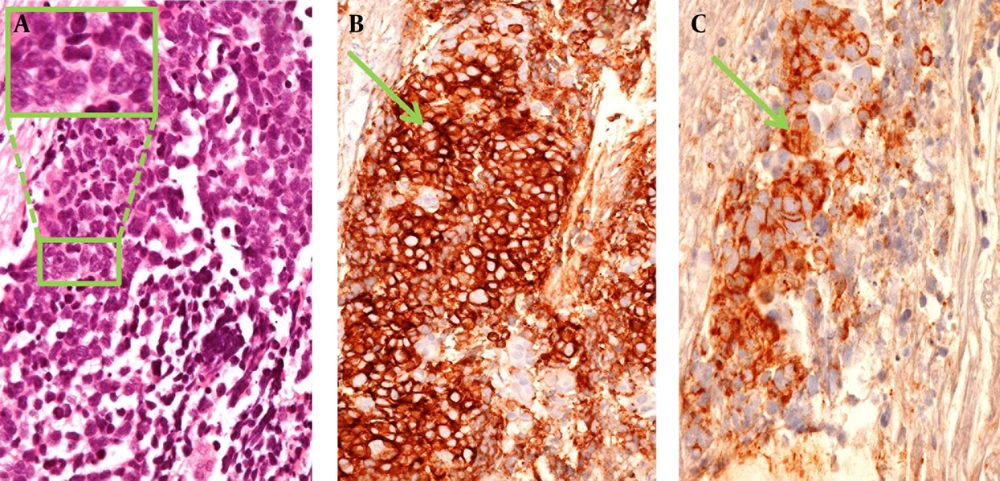
Our results can be outlined as follows: first of all, there is no leucocyte infiltration within the tumor or at its borders. Particularly an immune reaction, supported by activated monocytes or lymphocytes, is absent.(Figure 1). Secondly, a lymphatic vascular network was not particularly textured inside the neoplastic tissue or in its proximity and there was no evidence of neo-lymphangiogenesis. Finally, the stromal network of the neoplastic tissue was scantly represented and no desmoplastic reaction was observed (Figure 1).
Core needle biopsy of SCLC, showing small round or oval cells with scant cytoplasm, finely dispersed chromatin, sometimes with prominent nucleoli (green insert), without evidence of tumor-infiltrating lymphocytes (A, hematoxylin/eosin, original magnification ×20). The neoplastic cells (green arrows) were immunoreactive for CD56 (B, original magnification ×10) and chromogranin (C, original magnification ×20), both neuroendocrine markers, witness of their origin from the Feyrter's bronchial cells, belonging to the APUD system.
5. Discussion
Our histological findings showed biological and clinical behaviors of SCLC, profoundly different from any other lung cancer histotype (8). The absence of immunological competent cells can be interpreted as a sign of tissue tolerance and of host acceptance towards SCLC, causing expanded subsequent diffusion. Same feature is found with carcinoid tumors, histo-genetically correlated to SCLC (9), for their common origin from neuroendocrine cells, belonging to the Amine Precursor Uptake and Decarboxylation (APUD) system. The APUD system consists of chromaffin secreting cells, with a coordination function between nervous and endocrine systems, widely diffused along all the respiratory and digestive tracts, with higher incidence of carcinoids and small-cell cancers.
Development of the paraneoplastic syndrome, which is common in patients affected by SCLC, can be considered a biochemical proof of its tissue compatibility. Secretion of the immunosuppressive factors, instead of pro-inflammatory molecules, may be suspected.
Lymphatic vessel invasion is considered a negative prognostic factor in lung cancers (10, 11). The same conclusion cannot be drawn for SCLC, where a complete absence of neo-lymphangiogenesis is emerged. The lack of an evident stromal network can be correlated with the speediness of this neoplasia cellular replication and its particular local aggressiveness. On the contrary, the lymphocytic reaction to NSCLC, develops in parallel, through its stromal architecture (12-15). The absence of tumor-infiltrating lymphocytes, not supported by a textured stromal network, can be considered a histological marker of SCLC, correlating with a poor prognosis.