1. Background
Cisplatin (cis-diamine dichloroplatinium) is one of the major standard antineoplastic drugs, which still has a central role in cancer chemotherapy (1, 2). However, its significant antitumor activity is often limited due to development of renal toxicity (3). Cisplatin and other related platinum-based drugs are widely used for treatment of testicular, head and neck, ovarian and cervical carcinomas as well as nonsmall cell carcinoma of the lung. The side effects of cisplatin including neurotoxicity, ototoxicity, nephrotoxicity, nausea and vomiting in normal tissues are the major limiting factors for the use of this anticancer drug (4). Experimental studies showed that free radical formation through an oxidative stress pathway was the basic factor for cisplatin nephrotoxicity. Free radicals impair the reabsorption action of the proximal convoluted tubule (PCT) for water, ions (Na+) and glucose (1). The ability of lining the epithelia of the PCT to accumulate cisplatin five times greater than the serum concentration explains cisplatin-induced nephrotoxicity (5). Cisplatin nephrotoxicity is mediated partly through production of reactive species. Cisplatin increases its activity, which in turn increases free radical formation and simultaneously decreases antioxidant production (5). Kidney is an essential organ for metabolism and elimination of toxic agents and drugs such as cisplatin, via conjugating these agents to glutathione (1, 3, 5). Conjugation to glutathione is a main detoxification mechanism in kidney; but in PCT cells, conjugation of glutathione and cisplatin is the first step in the enzymatic pathway, which converts some intermediate compounds to potent nephrotoxin (6). Treatment of patients with cancer with chemotherapeutic drugs complicates the patient care and outcome due to likelihood of renal dysfunction. The extent of kidney damage varies with the type of chemotherapy, the type of malignancy being treated, age of the patient, and the underlying disease (5). The S3 segment of the long looped nephron of the coticomedullary region shows the highest degree of cell injury in cisplatin-induced renal toxicity, although the loop of Henle and the distal tubular segment can also be affected (5). Studies showed that at least 30% of patients treated with cisplatin, developed signs of renal dysfunction after a single dose injection of drug. Induction of apoptosis in renal tubular cells through Fas/FasL led to acute kidney injury, associated with a high mortality rate and need for renal replacement therapy (7). It seems that the patterns of epithelial alterations in kidney tubules are predominant of the segmental or focal type rather than the diffuse type (8).
2. Objectives
The histopathological aspects of renal toxicity after treatment of patients with chemotherapeutic agents have been the focus of intense investigations for many years. Hence, the aim of the present study was to evaluate the cytotoxic effects of single dose injection of cisplatin on rat, as an experimental model.
3. Patients and Methods
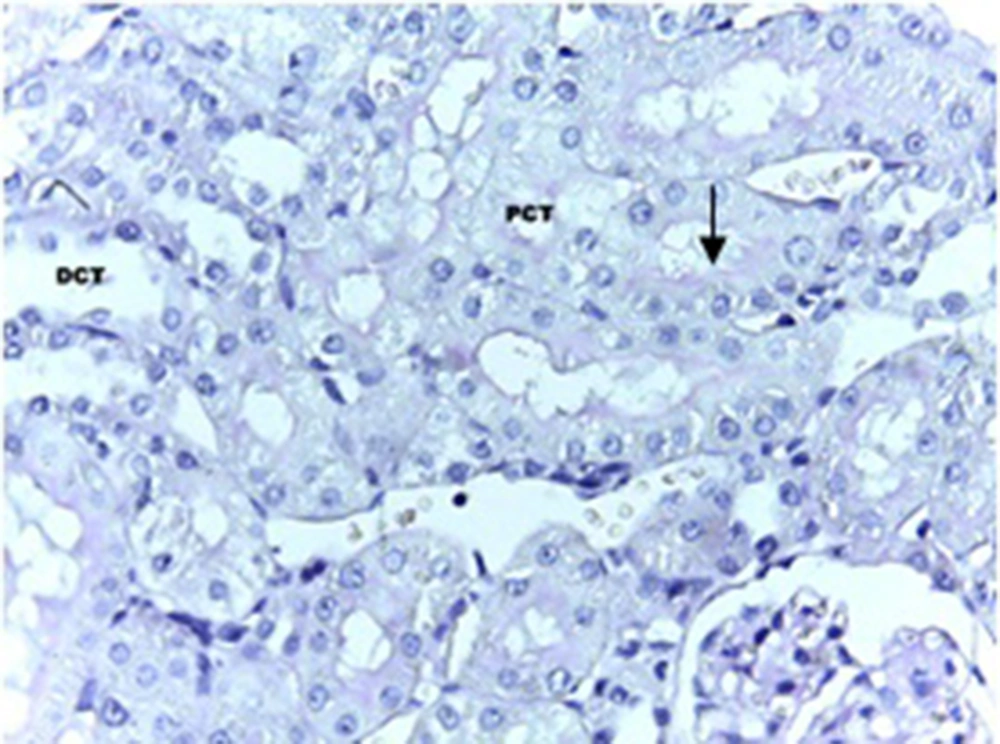
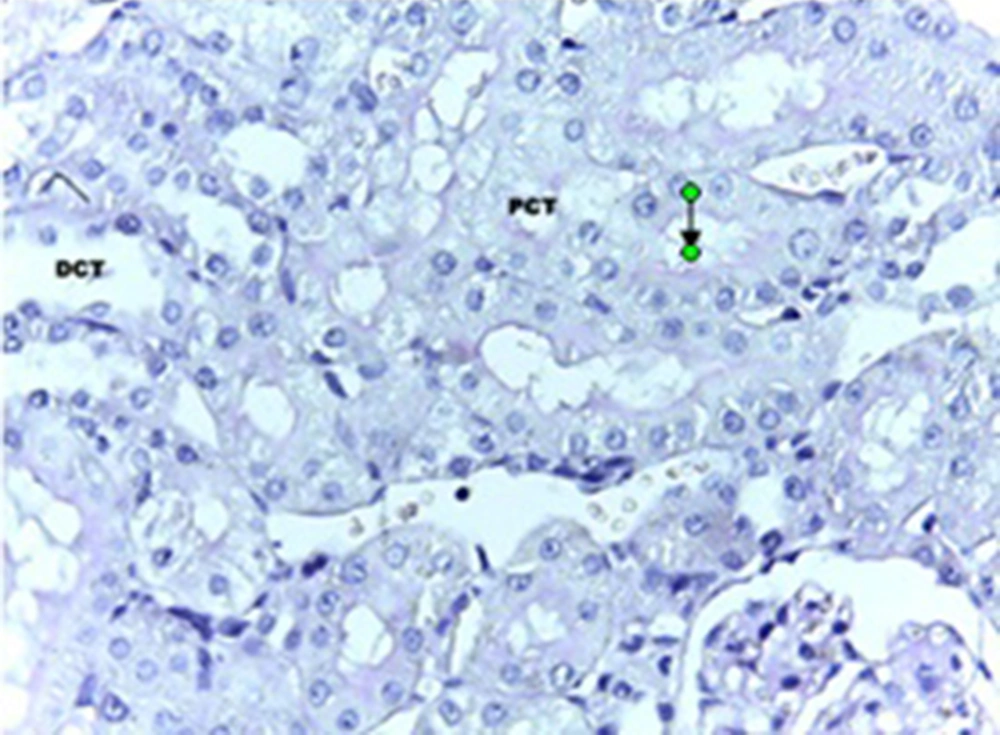
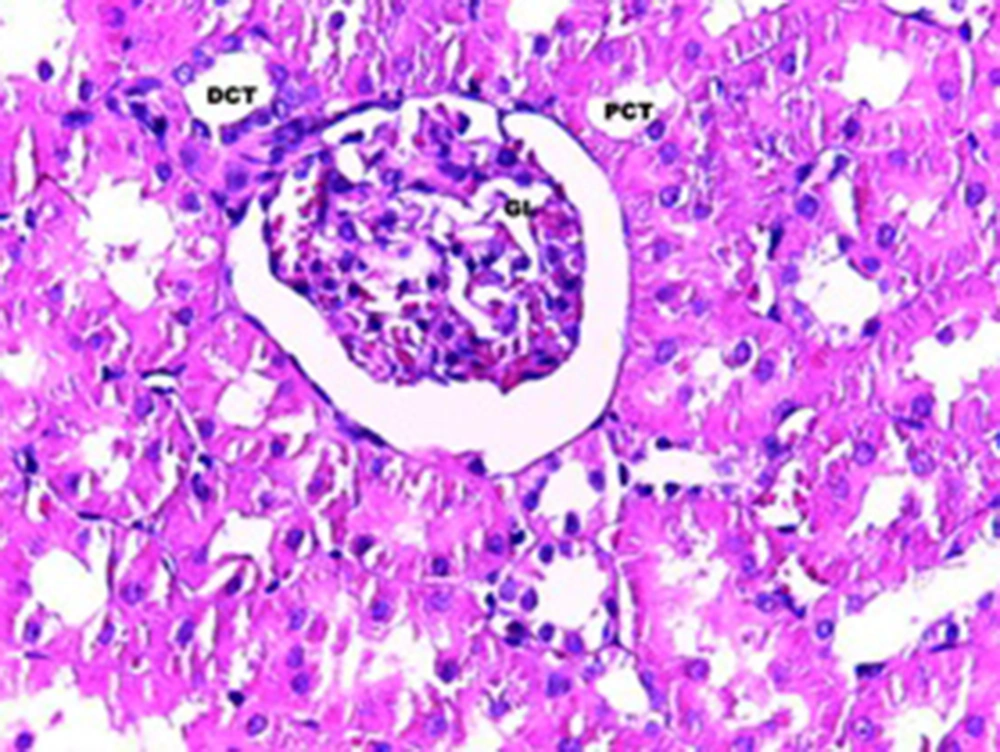
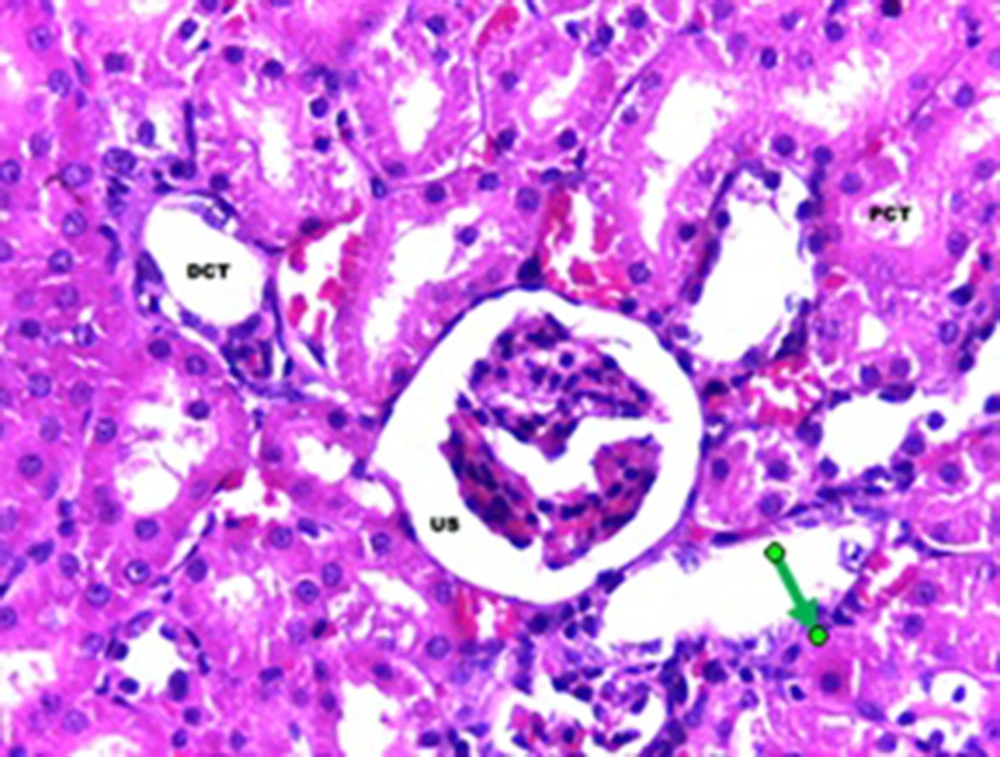
A total of 20 adult male Sprague-Dawley rats, purchased from Razi Institute, weighing about 210-230 g, were maintained under standard and identical conditions in the animal house of Zahedan University of Medical Sciences and received standard food pellets and water ad libitum (12 hours light /12 hours dark, 22 ± 2°C temperature and 40-45% humidity). The rats were divided into two groups including experimental (n = 10) and control (n = 10 among which five rats received the same volume of normal saline; five rats did not receive injection). The experimental rats were injected with 5 mg/kg of cisplatin, purchased from MYLAN Company, USA. A code was assigned to each rat, each of which was placed in one cage, and they were weighted before and after the experiment. At the end of the experiment after one week, rats of the experimental and control groups were given ether anesthesia and scarified. The kidney was rapidly removed and weighted. The tissue samples were processed routinely and the 6-µm sections were stained with hematoxylin-eosin and periodic acid-Schiff (PAS) methods. The histology sections were observed under a light digital photo microscope equipped with suitable software (Las Ez Ver 2.1.0). The diameters of the urinary space and the epithelial thickness of proximal and distal convoluted tubule were measured. The histological studies results were checked for any histopathological change. In each kidney samples of experimental and control groups at least 100 samples (proximal and distal convoluted tubules and urinary space) were measured. The collected data was analyzed with Mann-Whitney non parametric test, using SPSS software (version 17). The research plan was approved by the Ethical Committee of Zahedan University of Medical Sciences (91-1071).
4. Results
The statistical analysis by ANOVA test showed that there were significant differences regarding the changes in the epithelial thickness of proximal and distal convoluted tubules between the experimental and control groups (P < 0.001). Although, the same test for the diameter of urinary space between the studied groups did not show any significant changes. Scheffe test showed that there was a significant difference regarding the kidney weight/total body weight between the control and experimental groups (P < 0.007) (Table 1). Histopathological evaluation showed some degrees of atrophy for glomerulus and eosinophilic and vascular degenerations in the experimental group in comparison with the control group. Focal and segmental tubular necrosis was obvious in the experimental group. In the kidney medulla, dilation of blood vessels, mainly vasa recta, with eosinophilic precipitation was recognized. PAS staining showed atrophy of microvilli (brush border) in the affected proximal tubule (Figures 1-4).
| Groups | Urinary Space Diameter, µm | Thickness of the PCT Epithelium, µm | Thickness of the DCT Epithelium, µm | Kidney/Body-Weight Ratio × 1000 |
|---|---|---|---|---|
| Experimental | 10.96 ± 1.77 | 6.84 ± 0.31 | 11.96 ± 0.29 | 4.96 ± 0.45 |
| Intact | 9.78 ± 1.65 | 14.57 ± 0.38 | 8.81 ± 0.26 | 4.29 ± 0.13 |
| Normal saline | 8.446 ± 2.36 | 13.585 ± 1.01 | 7.957 ± 0.72 | 3.57 ± 0.63 |
5. Discussion
Our results showed histological change in proximal and distal convoluted tubules, which were signs of tubular necrosis and atrophy of the vascular component in glomerulus. These findings were in accordance with results reported by Ravindra and colleagues (1). In the ultrastructural level, cisplatin induced dense chromatin formation in the nucleus absence of microvilli in some proximal convoluted tubules and increased mitochondrial density and formation of rounded cisternae of smooth endoplasmic reticulum (1). Nephrotoxicity is the main limitation factor for treatment of patients with cancer. The mechanism of cisplatin nephrotoxicity is thought to be the formation of cisplatin-DNA complex. High doses of cisplatin induce focal tubular necrosis, although low doses induce apoptosis through caspase-9-dependent pathway (6). Our results showed some structural changes in glomerulus, which were obvious for the urinary space change in the experimental group. This finding was in accordance with the results of Yao et al. (3). Studies showed that accumulation of cisplatin in kidney tissue cells is the basic for cisplatin-induced nephrotoxicity. Cisplatin in the metabolization pathway converts to a reactive thiol, which is the potent nephrotoxin. It seems that in patients with acute renal failure, acute focal necrosis of proximal convoluted tubule is the predominant histological finding. The severity of necrosis and its patient outcome are dose-, concentration- and time-dependent (3). Acute renal failure is one of the best known complications of cisplatin administration in cancer patients. It seems that inflammatory cells and inflammatory cytokines are a portion of the mechanistic pathway in cisplatin-induced acute renal failure. The results of Fouble and coworkers showed that in cisplatin-induced acute renal failure, the extent of neutrophils as well as some inflammatory cytokines such as interleukin 1-β, interleukin 18 and interleukin 6 were increased in the renal tissue (9). Experimental studies showed that cisplatin-induced nephrotoxicity was mediated through tumor necrosis factor α, nitric oxide, intracellular adhesion molecules, and CD4+ regulatory T cells (10). Newer platinum agents such as carboplatin, oxaliplatin and nedaplatin seem to be less nephrotoxic than cisplatin and be considered as potential alternatives for treatment of patients with cancer as well as individuals at high risk of renal failure (5). Our results for induction of cisplatin-induced tubular necrosis and eosinophilic degeneration were in accordance with those of Safirstain and coworkers (10). Experimental studies on rat show that accumulation of platinum is mainly in the cytosolic compartment, although the process by which cisplatin enters the renal tubular cells is largely unknown. Furthermore, reduction of platinum uptake in tubular cells decreased renal toxicity (11). Our results on morphometric variables were in accordance with those of Agarwal et al. which showed a higher intensity of tubular necrosis after five days of cisplatin injection in rat compared with the control group (12). Our results showed significant changes in kidney/total weight ratio between experimental and control group which is in accordance with another study by Fouad et al. (13).