1. Background
Formaldehyde, an economically important chemical, is classified as a human carcinogen that causes different damages leading to cancer and probably leukemia (1). It is a gas at room temperature but it dissolves easily in water (2). It is used for fixation of tissues, aromatization and in other chemical components (3). It is found in all body cells and results from cerine, glycine and methionine metabolism (4). Different studies have indicated that it can produce free radicals in the body, which can cause harmful effects on different body organs (5, 6). Antioxidants are effective in removing the adverse effects of active components of formaldehyde. Vitamin E, C and A are antioxidants. Vitamin E has a main role in protecting the body against harmful effects of metabolically active types of oxygen that can have a strong antioxidant function (7, 8). Vitamin E can protect body organs such as the kidneys (9). It has been shown that vitamin E can suppress the damage of free radicals in cell membranes (10) and improve antioxidant enzyme activity in kidney tissues (11).
The urinary system has a major role in termination of toxic materials in most routine protocols. It has a major role in filtration, metabolism and deification of xenobiotic or their metabolic products (12). Chemical materials or their active metabolic types can transfer from plasma to kidney tubules and compared to other tissues can have a manifold concentration. Kidneys should receive 25% of heart output in order to have a good distribution of chemical materials (12).
2. Objectives
This research aimed to study the protective effect of vitamin E on kidneys damaged by formaldehyde.
3. Materials and Methods
A total of 24 adult wistar male rats weighing 250-300 grams were obtained from the Razi Institute (Tehran, Iran) and divided into three different groups (eight in each group). At first, animals were randomly assigned into three groups: Group A (control), Group E1 (formaldehyde), Group E2 (vitamin E and formaldehyde). The animals were housed under standard lighting conditions (12-hour light-dark cycle and room temperature of 27°C ± 1°C) with free access to water and food. They were allowed to adapt for at least one week to the animal room before they were subjected to treatment. Animals were maintained and handled according to the protocols approved by the Guilan University of Medical Sciences, Animal Care and Use Committee. Group A (control): rats received 1cc of intraperitoneal normal saline. Group E1 (Formaldehyde) received 10 mg/kg of intraperitoneal formaldehyde and Group E2, received 10 mg/kg of formaldehyde and 30 mg/kg of intraperitoneal vitamin E for two weeks. At the end of the experiments and 24 hours after receiving the last dose of vitamin E and formaldehyde, rats were sacrificed by cervical dislocation. Then the abdomen was opened to dissect the kidney, which was fixed in 10% formalin. For dehydration, kidneys were placed in ethyl alcohol and the tissues were passed through a successive series containing 30%, 70%, 80%, 90%, 95% and absolute alcohol. Then the tissues were cleared in xylenol and embedded in paraffin wax. Sections of 5 µm thickness were cut using “SLEE” rotator microtome (Germany). At least 5 slides from each kidney were stained with hematoxylin and eosin (H&E) for routine histological examination using a light microscope (13). The histological studied parameters of each slide included: Bowman's capsule, proximal and distal tubules. All above-mentioned parameters were studied morphologically. In each animal five slides and in each slide five microscopic fields were evaluated for histological studies and were classified into two grades of 1 and 2. Grade 1: high eosinophilic cytoplasm; long height; number of nucleus in each Bowman's capsule or between proximal and distal tubules; lack of congestion of blood vessels inside the Bowman's capsule or between tubules; lack of inflammatory cells, inside the Bowman's capsule or between tubules. Grade 2: light eosinophilic cytoplasm; short height; change in number of nucleus in each Bowman's capsule or between proximal and distal tubules; presence of congestion of blood vessels inside the Bowman's capsule or between tubules; presence of inflammatory cells, inside the Bowman's capsule or between tubules.
3.1. Statistical Analysis
For statistical analysis we used the SPSS software (version 16). Pearson chi-square and fisher tests were used and the statistical significance level was set to P < 0.05.
4. Results
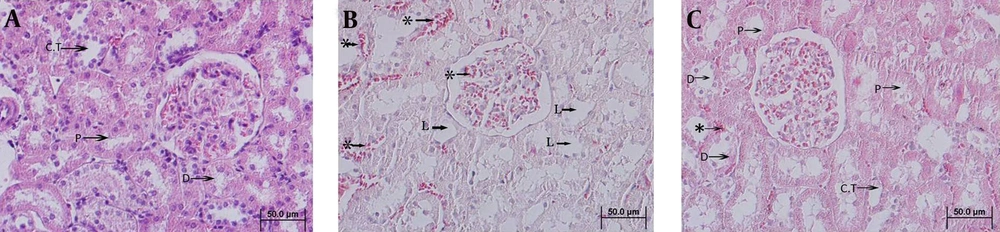
Bowman's capsule, proximal and distal tubules were studied in the control group. Bowman's space and capillary plexus were normal. The tubules had normal morphologically and eosinophilic cytoplasm or congestion of blood vessels or inflammatory cells inside the Bowman's capillary plexus or between proximal and distal tubules was not seen. Proximal tubules had a slightly larger outer diameter than distal tubules. Proximal tubules had a brush border and a star shaped lumen; whereas the distal tubules had a cleaner sharper luminal surface and fewer nuclei (Figure 1 A). Eosinophilic cytoplasm of Bowman’s capsule was statistically significant in three groups (P ≤ 0/05), but there were no significant differences in number of nucleus and height of cells in different groups (Figure 1) (Table 1). Comparison of eosinophilic cytoplasm, cell degeneration and lumen size of proximal tubules in three groups showed statistically significant differences (P ≤ 0/05) (Table 1); however, there were no statistically significant differences in the number of nucleus in this part of the kidney. In addition the lumen size and cell degeneration between the control group and E1, and between E1 and E2 were significant (P ≤ 0/05). The same parameters between control and the E2 group were not significant. Cell degeneration, lumen size, number of nuclei of distal tubules in all three groups showed significant differences (P ≤ 0/05). There were statistically significant differences in cell degeneration, lumen size, number of nuclei between the control and E1 groups (P ≤ 0/05) (Figure 1). Degeneration levels of cells between the control group and E2 showed statistically significant differences (P ≤ 0/05). However, there were no significant difference in the number of nucleus and the size of the distal tubules.
A) control (Group I) showing normal architecture; B) formaldehyde (Group E1) showing abnormal architecture such as: reduced eosinophilic cytoplasm, number of nucleus, short height, and presence of congestion of vessels; C) formaldehyde and vitamin E (Group E2) showing a relative regenerative architecture of Bowman's capsule. CT, collecting tubule; P, proximal tubule; d, distal tubule; L, lumen; *, congestion of vessels; H and E staining (× 400).
| Cell Height in PT | Congestion of Vessels | Cell Degeneration | BC Eosinophilic Cytoplasm | Nucleolus in PT | Lumen Size in DT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | |
| Control | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 | 0 |
| E1 | 1 | 6 | 2 | 5 | 1 | 6 | 5 | 2 | 2 | 5 | 2 | 5 |
| E2 | 8 | 0 | 7 | 1 | 2 | 6 | 8 | 0 | 6 | 2 | 8 | 0 |
| P Value | 0.000 | 0.004 | 0.000 | 0.082 | 0.01 | 0.001 | ||||||
a Abbreviations: BC, Bowman's capsule; DT, distal tubules; PT, proximal tubules.
5. Discussion
The results of this study revealed that administration of vitamins E can reduce the harmful effects of formaldehyde and improve the adverse changes on rat renal tissue in the treated group compared to the control group. Similar to our research, a study by Johannsen et al. and Til et al. also suggested specific pathological changes in renal tissue that was exposed to formaldehyde in their experimental groups (14, 15). These researchers stated that the severity of tissue changes depended on the amount and duration of exposure to formaldehyde. Studies on the effect of formaldehyde on the human body and its rapid metabolism (16), suggest that cellular toxicity of formaldehyde is probably due to its major metabolite, formic acid. Formic acid is excreted through two major routes, metabolism in the liver and excretion in the urine. Since formic acid metabolism in the liver is primarily saturated, exposure to a certain amount of formaldehyde and consequently formic acid can result to saturation of the enzymes. Thus, higher levels of formaldehyde exposure increases the concentration of formic acid in the liver cells and consequently in the plasma. Increased plasma concentrations of formic acid can cause primary metabolic acidosis and eventually systemic acidosis which results in renal excretion of formic acid (12). On the other hand formic acid inhibits cytochrome oxidase and cellular respiration cycle and increases anaerobic respiration that causes production of lactic acid; it also reduces the formic acid secreted into the renal tubules (17). Thus, an increase intracellular concentration in renal cells and serum will follow. Therefore, exposure to high concentrations of formaldehyde causes increasing formic acid concentration resulting in cell toxicity effects on the kidney. Formaldehyde can also cause the production of free radicals and the presence of antioxidants such as vitamin E can reduce the side effects of this substance. In this study, simultaneous treatment with vitamin E in the group receiving vitamin E and formaldehyde, greatly compensated the harmful effects of formaldehyde on the renal tissue. The present study results as well as the results of previous studies showed that vitamin E as an antioxidant can reduce reactive oxygen species (ROS) levels in the kidneys (11). Vitamin E as a powerful antioxidant and is the first line of defense against the peroxidation of fatty acids in phospholipids of cell membranes (18). In addition, lipid peroxidation index in the presence of vitamin E reduces (19). Vitamin E is also a powerful antioxidant that maintains the permeability of fluidity of biological membranes and prevents them from demolition (19). Vitamin E may increase the expression of E-cadherin in kidney tissue and renal tubular basal membrane degradation can be improved (20). Factors such as carboxyethyl hydroxychromans (CEHC) are water-soluble metabolites of vitamin E that have anti-inflammatory and antioxidant properties. These metabolites increase in progressive renal diseases and improve renal function (19). Given the protective role of vitamin E against oxidative damage in the kidney (21) and other study results, it has been accepted that vitamin E has antioxidant properties and elevates the levels of catalases in kidney tissue (11). Considering that these enzymes are responsible for the body's defense against free radicals (21), vitamin E has greatly improved the harmful effects of oxidative stress created by formaldehyde in the kidney tissue. The results of this study indicate that vitamin E may largely improve the adverse effects of formaldehyde on rat kidney tissue. Thus considering that these contaminants exist in everyday dietary consumption, and that human will inevitably be exposed to these environmental contaminants, a greater consumption of vitamin E is recommended.