1. Background
Fish comprise around half of all vertebrate species together, yet very few cell lines have been established and characterized with specific biomarkers from piscinid species in comparison to mammals. Cell line research has gained momentum in the last decade and a number of cell lines from different organs of different fish species have been established in India, such as the SICH cell line from heart of Catla catla (1); two cell lines, RE and CB from the eye of Labeo rohita and brain of Catla catla, respectively (2); three cell lines RF, RH and RSB from heart, fin and swim bladder of Labeo rohita, respectively (3); cell lines from the fin tissue of Tor tor (4); two cell lines from fin and eye tissue of Tor chelynoides (5, 6) and fin tissue of Scizothoraxrichardsonii (7). These in vitro cell culture systems have proved to be essential tools for studying cellular biology, biotechnology and toxicology (8-11). Fish cell lines have been successfully used to evaluate the cytotoxicity potential of more than 50 aquatic pollutants, such as heavy metals, pesticides and nanoparticles (12). In vitro data obtained from fish cell lines have shown good correlation with in vivo toxicity data.
Wallago attu is a natural denizen of Indian subcontinent. It is a large freshwater fish with majestically elongated body and a highly nutritive flesh. Development of a cell line from this important species will help in germplasm conservation and in studying fish biology and the impact of potential toxicants on this fish species.
2. Objectives
The present study reports the establishment and characterization of a novel muscle cell line from Wallagoattu. The paper also investigates cytotoxicity potential of organophosphate pesticides, chlorpyrifos and malathion, on the established cell line.
3. Materials and Methods
3.1. Development of Primary Culture
Healthy fingerlings of W. attu (4-8 cm) were acclimatized at the wet lab facility of National Bureau of Fish Genetic Resources (NBFGR), Lucknow, India. Fingerlings were starved for 24 hour, disinfected with 70% ethanol and euthanized in ice before explant preparation. Muscle tissue was aseptically excised, washed [phosphate buffered saline (PBS) containing 5x of antibiotic-antimycotic solution)] and chopped to explants of 1-2 mm2 size. Explants were seeded in 25 cm2 of fetal bovine serum (FBS) treated tissue culture flasks. After 24 hours of incubation at 28°C, L-15 medium containing 20% FBS and 10 ng/mL of basic fibroblast like growth factor were added. The flasks were regularly monitored for attachment, growth, division and migration of cells from explants, using a phase contrast inverted microscope.
3.2. Establishment of Cell Line and Maintenance of Culture
After the primary culture reached 90% confluency, the cells were trypsinized using 0.05% trypsin solution and 0.2% ethylenediaminetetraacetic acid (EDTA) and were subcultured with a split ratio of 1:2. Cells were subsequently maintained with regular subculturing at an interval of 5-7 days.
3.3. Growth Studies
The cell line was characterized with regards to the optimal growth requirements of the cells. Optimal temperature, FBS and basic fibroblast growth factor (bFGF) were calculated at 20th passage. Briefly, the 1 × 105 cells were seeded in 25 cm2 tissue culture flasks and were incubated at 18, 20, 24, 28 and 32°C for 1 week. Cells from one flask at each of the mentioned temperatures were harvested and counted using a hemocytometer every day during the week. Similarly, cell growth studies after supplementing the media with different concentrations of FBS (5, 10, 15 and 20%) and bFGF (0, 5 and 10 ng/mL) were performed at 28°C. All the experiments were done in triplicates and the results are expressed as arithmetic mean ± standard error (SE).
3.4. Immunocytochemistry
Cellular morphology was confirmed using monoclonal antibodies, which were directed against vimentin protein at the 50th passage. Cells at 70% confluency were rinsed with PBS and fixed in methanol for 30 minutes at room temperature. The fixed cells were then washed with PBS; permeabilized in 0.1% Triton X-100; blocked in PBS containing 5% sheep serum and incubated for 60 minutes at 28°C. The blocking solution was discarded and 100 µL of primary antibody i.e. 1:40 dilution of anti-vimentin clone V9 (V6630-CLONE 9 Sigma) were added in duplicate chambers and incubated for 18 hours at 4°C. The cells were then washed with PBS and reincubated for 45 minutes with 100 µL of 1:250 dilution of fluorescein isothiocyanate (FITC)-labeled anti-mouse IgG. Cells were finally rinsed in PBS, mounted with 50% glycerol (in PBS) and observed under the fluorescence microscope. Suitable controls for autofluorescence and secondary antibodies were used.
3.5. Chromosomal Analysis
Chromosomal analysis of the cell lines was carried out at different passage. Briefly, cells grown to 90% confluency in 75 cm2 tissue culture flasks were treated with colchicine solution (1 μg mL-1) for 3 hours. Treated cells were harvested using centrifugation (1200 g for 5 minutes). The resulting pellet was treated with 0.56% KCl solution for 10 minutes and the cells were fixed in Carnoy’s fixative. Metaphase slides were prepared using the drop splash technique; chromosomes were stained with 5% Giemsa for 10 minutes and were analyzed using 100X oil immersion objective lens microscopy (13).
3.6. Molecular Characterization
Molecular authentication of the cell line was carried out using sequence amplification and analysis of mitochondrial 16S rRNA and cytochrome oxidase subunit I (COI) gene using the protocols described by Goswami et al. 2012 (5).
3.7. Gene Expression Studies
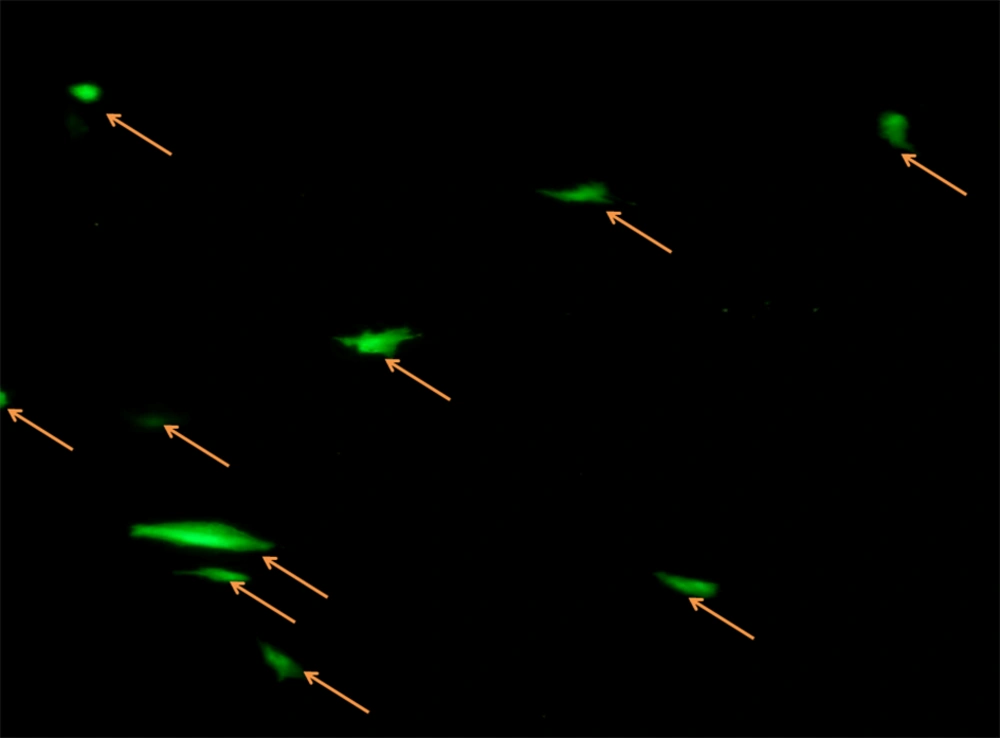
The 70-80% confluent Wallago attu muscle (WAM) cells were transected with pEGFP-C1 plasmid at 40th passage using Lipofectamine® LTX and PLUS™ reagents (Invitrogen, Waltham, Massachusetts, The USA). The plasmid (200 ng of pEGFP-C1) was mixed in 100 µL of Opti-MEM media to which 0.5 µL of PLUS™ reagent was added. The amount of 2 µL of Lipofectamine® LTX was added to the mixture, which was incubated for 30 minutes at 28°C. The mixture was added dropwise to 70% confluent WAM cells. The medium was discarded after 6 hours and fresh growth medium was added. Cells with green fluorescence were observed within 24 hours under a fluorescence microscope.
3.8. Pesticide Toxicity on WAM Cell Line
The WAM cell line was used as an in vitro model for cytotoxicity evaluation of two organophosphates viz. chlorpyrifos and malathion. Cytotoxicity was assessed using methylthiazol tetrazolium assay (MTT) reduction and neutral red (NR) uptake assay in 96-well plates. Test chemicals of varying concentrations (100, 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78125, 0.390625 mg/L) were prepared in L-15/ex solution and were added to wells with 90% confluent monolayers for 24 hours. All the pesticides were tested in six replicates per microplate. Ten µL of MTT solution (5 mg/mL) were added in all wells and the plates were incubated for 4 hours at 28°C. After incubation, the MTT containing medium was replaced with dimethyl sulfoxide (DMSO). The plates were shaken on the gyratory shaker for 10 minutes and the absorbance was recorded at 570 nm wavelength.
Neutral red uptake assay was performed by incubating the pesticide exposed cells with 100 µL of 33 µg/mL of neutral red solution (prepared in L-15/ex solution) at 28°C for 2 hours. The cells were washed with NR fixative solution and the lysosomal NR was extracted in 100 µL/well of NR extraction solution. The plates were shaken on the gyratory shaker for 10 minutes and the absorbance was recorded at 530 nm wavelength.
3.9. Statistical Analysis
All the cytotoxicity experiments were performed in triplicates. The dose response curve was prepared using Graph Pad Prism 5 (GraphPad Software Inc., La Jolla, CA, The USA). The individual data points of the concentration-response cytotoxicity charts were presented as the arithmetic mean percent inhibition relative to the control ± standard deviation (SD).
3.10. Cryopreservation
Revival efficiency of cells following liquid nitrogen (LN2) storage was evaluated in cryopreservation medium (25% FBS and 5% DMSO in L-15 Medium) at 20, 30, 40 and 50 passages. Exponentially growing cells were harvested by centrifugation and the cell pellet was washed with PBS and mixed in L-15 medium with 20% FBS and 10% DMSO. Cells were diluted to 3 × 106 cells per mL; aliquots of 1 mL were added to cryovials (10 numbers); were kept in Mr. Frosty freezing container (Thermo Fisher Scientific, Waltham, MA, USA) and were incubated overnight at -80°C. The vials were then transferred into liquid nitrogen containers (-196°C). After storage for 6 months, one vial for each batch was quickly thawed and the cryopreservation medium was removed through centrifugation. The cells were resuspended in growth medium and seeded into 25 cm2 tissue culture flasks. The number of viable cells was counted using an hemocytometer after staining with trypan blue.
4. Results
4.1. Establishment of Cell Line
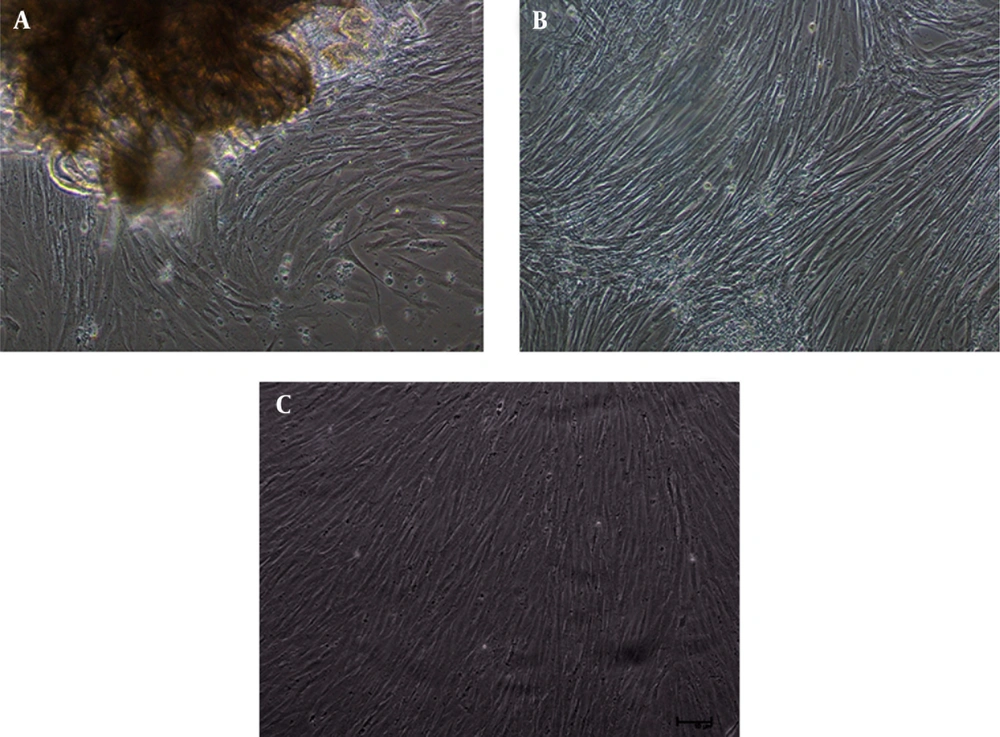
Radiation from muscle explants started after 10-15 days of explants attachment. A confluent monolayer was observed after 18-22 days of explant preparation (Figure 1 -A). The cell line has been successfully maintained over 50 passages in L-15 medium supplemented with 20% FBS (Figure 1 B and 1 C). The cell line was subcultured with a split ratio of 1:2, which was reduced to 1:3 by the 20th passage.
4.2. Growth Studies
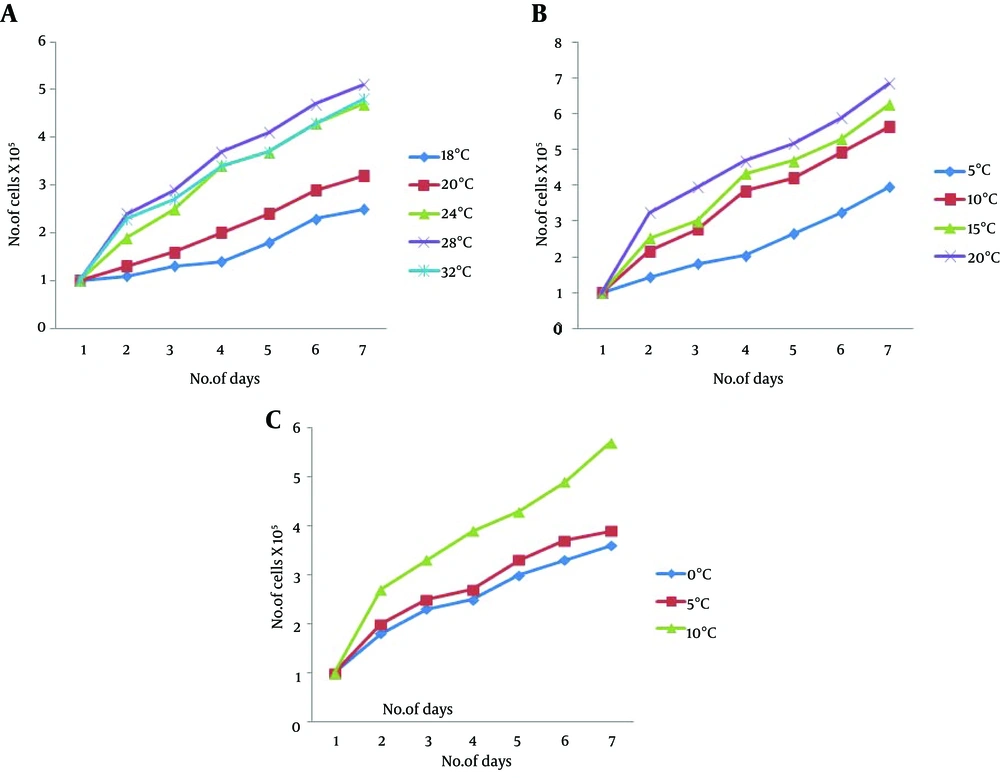
The growth of cells revealed an increase in cell number as the culture temperature increased from 18 to 28°C. However, the optimum growth was observed at 28°C (Figure 2 A). Similarly, the optimal growth concentration of FBS was 20% at 28°C (Figure 2 B) and bFGF was 10 ng/mL (Figure 2 C).
4.3. Immunocytochemistry
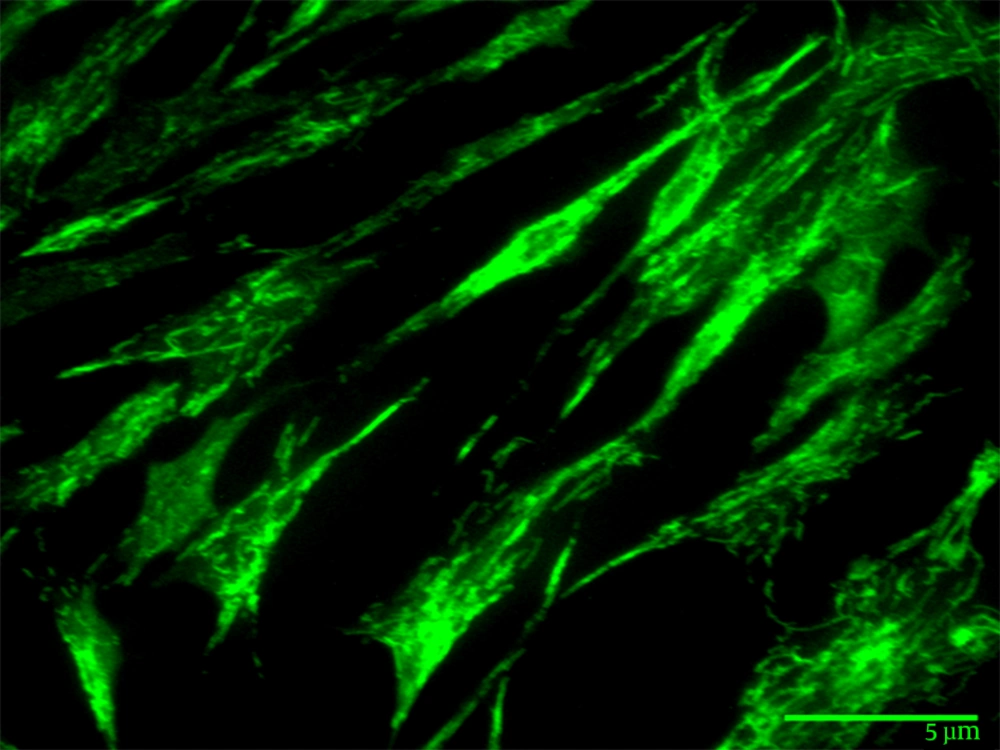
Morphological observation by optical microscopy revealed fibroblast like cells in the culture. This result was validated by the immunocytochemistry of WAM cells, as these cells gave positive staining reaction to monoclonal antibodies directed against vimentin (Figure 3).
4.4. Chromosomal Analysis
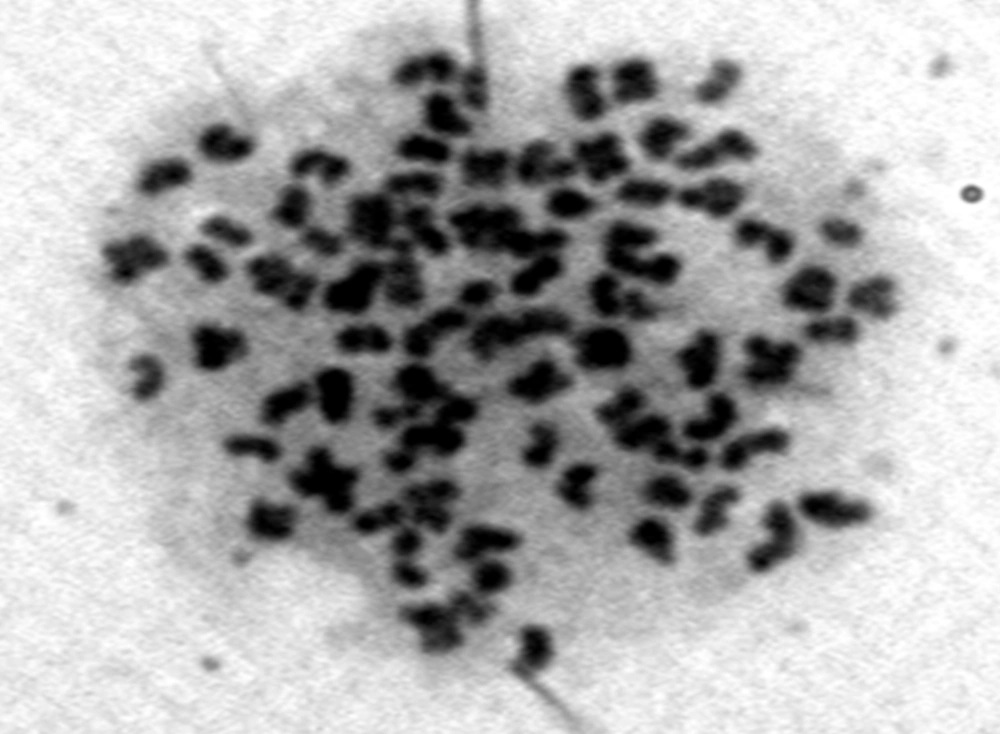
Chromosomes counts of 62 metaphase slides from WAM cell line revealed 66 to 88 diploid chromosomes with a model value of 86 (Figure 4). The deviation from modal value could be accorded to the loss or gain of chromosomes during slide preparation. Although both aneuploidy and heteroploidy were observed, their number were significantly lower.
4.5. Molecular Characterization
Analysis of mitochondrial 16S ribosomal RNA and COI genes was performed to authenticate the origin of the cell line. Sequence alignment of COI and 16S rRNA genes derived from WAM cells demonstrated 99-100% similarity with the known mitochondrial DNA sequences of W. attu voucher specimens submitted previously to Genbank, National Center for Biotechnology Information NCBI.
4.6. Transfection Efficiency
The WAM cell line was successfully transfected with the pEGFP-C1 plasmid using the Lipofectamine® LTX and PLUS™ reagents (Invitrogen, Waltham, Massachusetts, The USA). The expression of the EGFP plasmid in the cell line was detected after 24 hours of transfection (Figure 5). The observed transfection efficiency of the cells was calculated to be 15% for WAM.
4.7. Cytotoxicity Studies With Organophosphate Pesticides
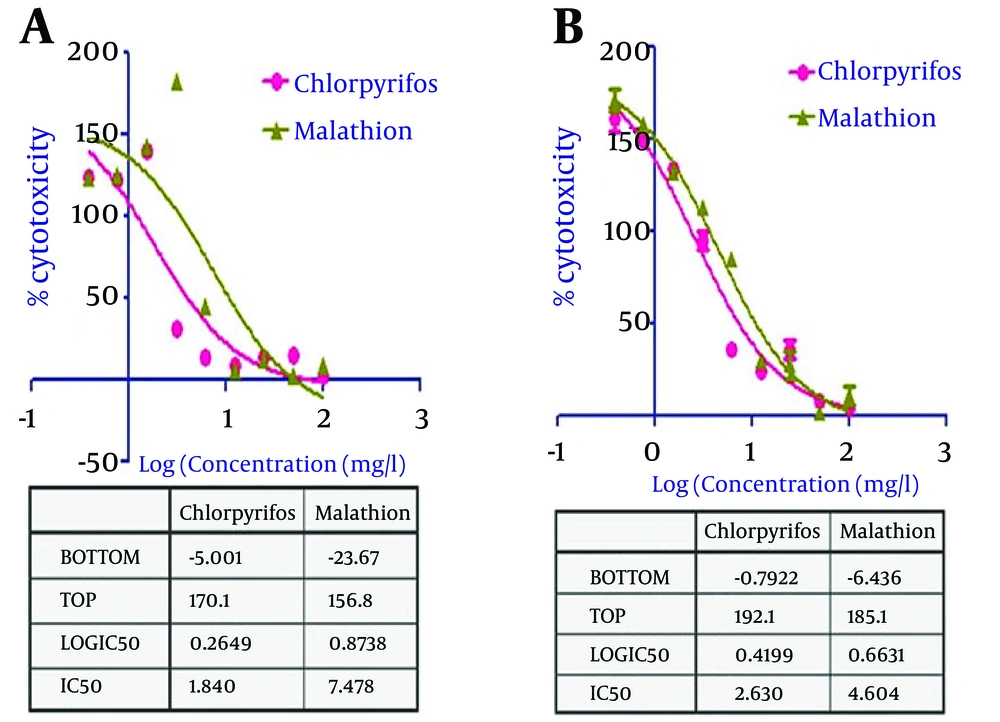
A significant dose dependent cytotoxic response was observed in the WAM cells upon exposure with test pesticides viz. chlorpyrifos and malathion (Figure 6). The IC50 values calculated from the dose response curve using MTT and NR assays were 1.840 ± 0.54 and 2.630 ± 0.67 mg/L, respectively, for chlorpyrifos. Similarly, IC50 values for malathion were 7.478 ± 1.18 and 4.604 ± 0.84, respectively (Table 1).
| Pesticides | Exposure Period and Endpoint | IC50, mg/L | |
|---|---|---|---|
| 1 | Clorpyrifos | 24 hour MTT assay | 1.840 ± 0.54 |
| 1 | Clorpyrifos | 24 hour Neutral Red uptake assay | 2.630 ± 0.67 |
| 2 | Malathion | 24 hour MTT assay | 7.478 ± 1.18 |
| 2 | Malathion | 24 hour Neutral Red uptake assay | 4.604 ± 0.84 |
a Abbreviations: MTT, methylthiazol tetrazolium assay; WAM, Wallago attu Muscle.
4.8. Cryopreservation
Recovery of the viable WAM cells stored in liquid nitrogen (-196°C) revealed the capability of the cryopreserved cells to survive 6 months of cryostorage. The cells revived successfully with an efficiency situated between 54-56%.
5. Discussion
The present study reports the successful development of a muscle cell line from threatened freshwater shark, W. attu. The primary culture was established using the explant technique which is advantageous over the trypsinization method in terms of speed, ease and maintenance of cell interactions (14). A large number of continuous cell lines has been developed by using the explant procedure (1, 2, 5, 15). The optimum physicochemical environment characteristics like culture medium, FBS concentration, growth supplements, incubation temperature, etc., vary considerably across fish species. The cells grew well at a wide range of temperatures from 18°C to 32°C, with an optimum value of 28°C. The results were in conformity with the other fish cell lines reported earlier (4, 5, 15). Growth at a wide range of temperatures makes the cell line suitable for the isolation of both warm and cold-water fish viruses (16). Suitability of L-15 in supporting fish cell lines, compared to that of other media, has been documented by Fernandez et al. (1993) which is designed to maintain the pH in the physiological range under normal atmospheric conditions without added CO2 (17). Most of the previous works preferred L-15 or Dulbecco's modified Eagle's medium (DMEM) medium to develop cell cultures from fish (5, 15).
The FBS is essential for survival and optimal growth of cells. In primary cell cultures, FBS at high concentrations (20%) is favorable for cell growth and proper attachment. The growth rate of the W. attu cells increased when the FBS concentration was augmented from 5% to 20%. A relatively good growth of the fish cell lines was observed at 10% FBS. However, maximum growth was observed with the concentrations of 15% and 20%. Human basic fibroblast growth factor (10 ng/mL) was supplemented with the growth medium for growth and maintenance of the cell line. Investigations into the use of bFGF did increase the growth of cells in fish primary cultures, although they were not essentially required. Chen et al. (2004) have reported increased cell growth due to supplementation of bFGF (18).
Species identification of cell lines is crucial for the accuracy and reproducibility of results in scientific research. The 16S rRNA and COI gene sequences derived from WAM cells demonstrated a 99-100% similarity with the known mitochondrial DNA sequences derived from W. attu voucher specimens of The National Center for Biotechnology Information (NCBI), which authenticates the origin of cell lines. Hebert et al. (2003) have demonstrated the utility of COI gene as a universal barcode, referred as “DNA barcoding”, for the genetic identification of animal life (19). The COI region has been consistently used by a large number of researchers for identification of cell lines (3-5, 15). The diploid status of a cell line is a good indicator of chromosomes stability, which may be relevant to the proximity of a cellular system to the original physiological condition. The established cell line was stable with a diploid chromosomal count of 86. The antibody against fibroblastic proliferative marker was used to confirm the nature of the cell line. Vimentin, which is a typical intermediate filament protein in the fibroblasts, was found to be expressed in WAM cells, which confirmed its fibroblastic nature. The estimated transfection efficiency was 15% for WAM, which is higher compared to that observed earlier with Puntius sophore caudal-fin (PSCF) cell line, with 10% efficiency (15) and also to other fish cell lines (20). This makes the cell line useful for conducting studies on functional genomics, such as RNA interference, gene knockout and pathogenesis studies against W. attu infectious agents, using this cell line. The cryopreserved cells exhibited a revival efficiency of over 50% after 6 months of cryopreservation, without any significant morphological alteration or changes in growth rate and cell doubling time. The viability of cryopreserved cell lines was more or less similar with that of other fish cell lines, as for the 73% for GF-1 (E. coioides) (21) and 75% for PSCF (15). Cryopreservation of cell lines is necessary to minimize genetic changes in continuous cell lines, long-term storage, and to avoid aging and transformation in cell lines (13). These cryopreserved cell line will be instrumental in conserving biodiversity of this important endangered freshwater fish species.
Fish cell lines have been extensively used as in vitro systems for cytotoxicity evaluation of aquatic pollutants (12). Cell viability assays are significantly affected by the exposure medium, and in this instance, the L-15/ex medium was used for the cytotoxicity assays. Absence of antioxidants in L-15/ex helps in expression of cytotoxicity caused by aquatic pollutants. Neutral red uptake and MTT reduction assays are routinely performed for cytotoxicity testing. The IC50 value, which is the concentration of pollutant (pesticides) required to induce cellular cytotoxicity in 50% of the cell population, was found to exhibit a dose dependent increase in cytotoxicity. Cowman and Mazanti (2000) have reported that 96 hours IC50 for chlorpyrifos ranges from 1 mg/L in Bufo americanus to 3 mg/L in Rana pipiens (22). For malathion, substantial mortality was observed in Bufo arenarum at 47.3 mg/L after 5 days. These organophosphates exert their toxicity through the inhibition of cholinesterase. While there may be ancillary effects of pesticides (23), cholinesterase depression remains the primary mechanism. Therefore, the present study strongly validated the utility of WAM cell line as an in vitro biological system for aquatic toxicity assessment of pesticides.