1. Background
Rheumatoid arthritis (RA) is an auto-immune, inflammatory, and systemic disease that destroys the joints (1). In this disorder, hands and feet are the most affected parts of the body (2). RA is nearly 3 times more likely in women than in men and its onset is more common between 40 and 60 years of age, but the disease may occur at any age. In developed countries, between 0.5 and 1% of adults, and in developing countries, five to 50 cases per 100,000 people are diagnosed each year (3, 4). The main cause of this disease is unknown; however, genetic and environmental factors are involved in the pathogenesis of RA (5). Genetic factors, especially HLA loci, are important genetic factors for susceptibility to RA. The HLA-DRB1 locus is one of the most important predisposing genetic factors for susceptibility to RA (6). The MTHFR gene encodes an enzyme called methylenetetrahydrofolate reductase, which participates in folate metabolism and in the remethylation of homocysteine to methionine (7, 8). Goyette et al. initially identified a single nucleotide polymorphism at position 677 in the MTHFR gene (7) that, in the heterozygous or the homozygous state (CT and TT genotypes), leads to reduced enzyme activity and increased thermolability or activity at high temperature (9).
2. Objectives
3. Patients and Methods
3.1. Study Population
This study included 120 RA patients and 120 healthy controls recruited from the Golestan hospital of Ahvaz, Khuzestan that were Arab and non-Arab (Bakhtiaris/Lurs). All patients met the American College of Rheumatology classification criteria for RA (13, 14). Demographic variables of patients with RA and controls are summarized in Table 1. The study was approved by the Ethics Committee of the Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, and was conducted according to the Helsinki declaration.
| Variables | Cases | Controls | P Value |
|---|---|---|---|
| Age, y | 45.3 ± 11.7 | 44.7 ± 9.6 | 0.814 |
| Ethnicity | 0.001 | ||
| Arabs | 73 (62.4) | 44 (37.6) | |
| Others | 47 (38.2) | 76 (61.8) | |
| Gender | 0.051 | ||
| Male | 23 (39) | 36 (61) | |
| Female | 97 (53.6) | 84 (46.4) |
a Values are presented as mean ± SD or No. (%).
3.2. Genotype Determination
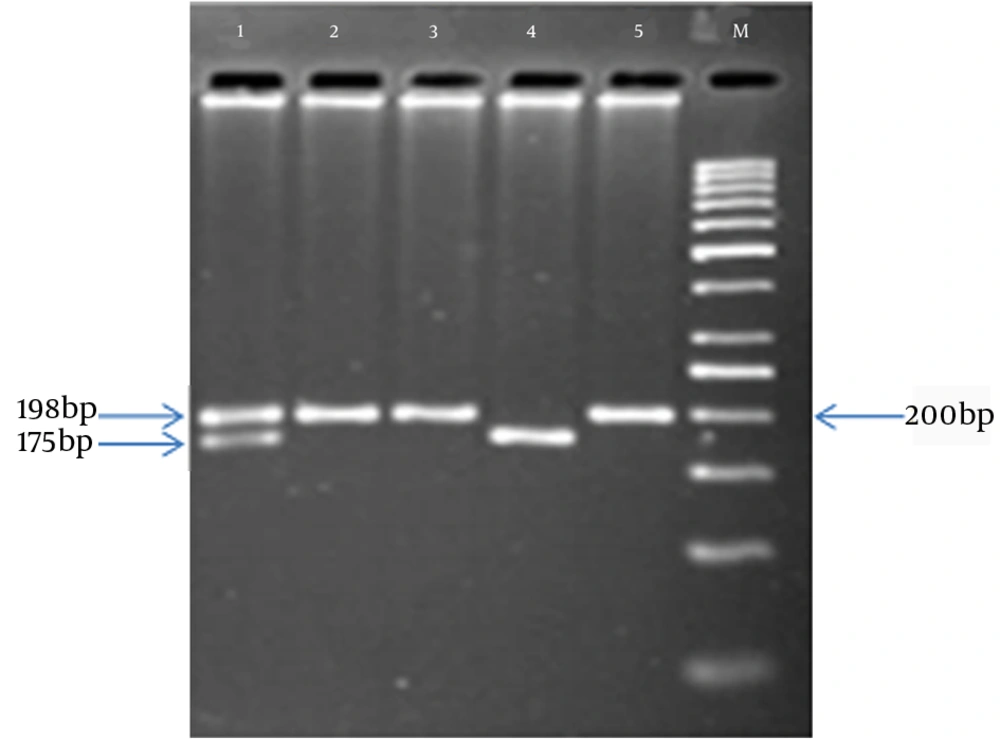
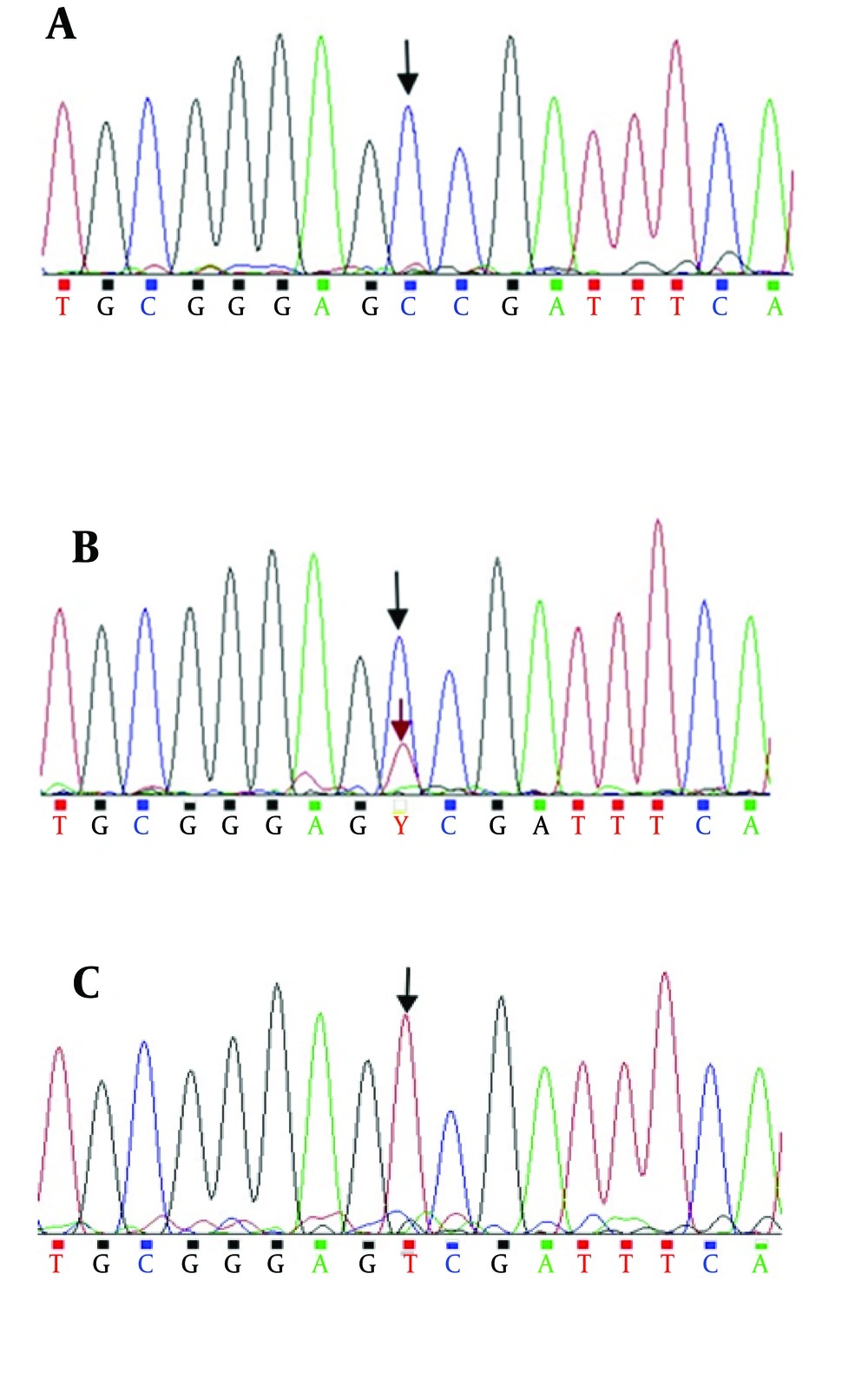
Genomic DNA was isolated from blood samples by the salting-out method (15) and the MTHFR C677T mutation (rs1801133) was analyzed by PCR-based RFLP methods (15). Amplification was carried out using the primers F5’-TGAAGGAGAAGGTGTCTGCGGGA-3’ and R5’-AGGACGGTGCGGTGAGAGTG-3’ with initial denaturation at 94°C for 5 minutes, 30 cycles of denaturation at 94°C for 30 seconds and annealing at 58°C for 30 seconds, and final extension at 72°C for 5 minutes (16). The PCR products were genotyped for the MTHFR C677T polymorphism by PCR-RFLP, and the PCR products subsequently were sequenced (Figure 1). The PCR products were digested with HinfI and then were separated on 2.5% agarose gels, stained with ProtoBlue Safe stain (Syngene, UK), and photographed on an ultraviolet transilluminator. The homozygous, wild type allele (CC) was identified by the presence of one 198-bp band. The heterozygous allele (CT) was identified by a 198-bp and a 175-bp band, while the homozygous, mutant allele (TT) was identified by a 175-bp band. Both the CT and TT alleles were predicted to produce a 23-bp band; however this band was not visible on the agarose gel (Figure 2).
3.3. Statistical Analysis
Statistical analysis of the data was performed using the statistical software, SPSS20.0 (IBM SPSS Statistics 20). The continuous data are given as mean ± standard deviation (SD) and the frequencies of the alleles and genotypes in patients and controls were compared using chi-square analysis. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated. P-values smaller than 0.05 (two-tailed) were considered statistically significant.
4. Results
Table 1 shows the relationship between the variables gender, ethnicity, and age, and RA risk. The mean age ± SD was 45.03 ± 11.7 in patients and 44.70 ± 9.6 in control group.
There were 97 (80.8%) women and 23 (19.2%) men in the patient group and 84 (70%) women and 36 (30%) men in the control group. Statistical analysis indicated no relation between age, gender, and RA risk. However, analysis indicated that RA risk in Arabs is higher than that in other ethnicities (P < 0.05). Table 2 shows the distribution and allelic frequencies of the MTHFR C677T polymorphism in the patient and control groups. Our data indicated that there are significant differences between RA patients and healthy controls in allele (P = 0.004) and genotype (P = 0.015) frequencies. We found that the CT genotype is associated with the risk of RA (P = 0.008) and compared with CC and TT genotypes, the CT genotype increases the risk of RA, while comparison of the TT with the CT and CC indicated no significant association between the TT genotype and the risk for RA. Table 3 shows the clinical factors, negative or posetive state of rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP) for the patient group.
| Variables | Patient a | Control a | P Value | OR (95% CI) | Adjusted OR (95% CI) b |
|---|---|---|---|---|---|
| Genotype | |||||
| CC | 61 (50.8) | 83 (69.2) | - | 1 | 1 |
| CT | 46 (38.4) | 29 (24.2) | 0.008 | 2.158 (1.220 - 3.818) | 2.028 (1.115 - 3.691) |
| TT | 13 (10.8) | 8 (6.6) | 0.098 | 2.211 (0.863 - 5.664) | 1.794 (0.676 - 4.757) |
| CC + TT | 59 | 37 | 0.004 | 2.170 (1.28 - 3.677) | - |
| TT versus others | 13 | 8 | 0.253 | 1.701 (0.678 - 4.267) | - |
| Alleles | 0.004 | ||||
| C | 168 (70) | 195 (81.2) | 1 | - | |
| T | 72 (30) | 45 (18.8) | 1.857 (1.213 - 2.843) | - |
a Values are presented as No. (%).
b The adjusted OR for age, ethnicity, and gender.
a These variables are just for the patient group and are presented as No. (%).
Comparison of either RF or anti-CCP with genotypes showed that there was no significant difference between these clinical factors and genotypes. We found no significant association between RF and anti-CCP. No obvious deviation from Hardy-Weinberg equilibrium was observed for the MTHFR C677T polymorphism in either the patients or the controls (P > 0.05). Finally, in this study, we observed no relation between genotype and allele frequencies for the different ethnicities (Table 4).
5. Discussion
Rheumatoid arthritis is a systemic, inflammatory disease. The cause of RA is not clear, but both genetic and environmental factors are involved in this condition. One of the genetic factors that has recently been considered for risk of RA is the MTHFR C677T (rs1801133) polymorphism. We studied 240 subjects (120 patients and 120 healthy controls) for genotyping of the C677T polymorphism. PCR products were sequenced to verify the presence of the polymorphism (Figure 1).
In this study, we examined the prevalence of the MTHFR C677T polymorphism among Khuzestan adults. The percentage of genotypes was 69.15% CC, 24.15% CT, and 6.7% TT in controls and 50.8% CC, 38.4% CT, and 10.8% TT in the patient group. Statistical analysis indicated a significant association between genotype and the risk for RA (P = 0.015).
We found no association of age, gender, or clinical factor (RF and anti-CCP) variables with RA. When we compared the relation between ethnicity and risk of RA, we found a statistically significant association. However, no relation was found between both RF and anti-CCP, and the risk of RA. The MTHFR C677T polymorphism is associated with reduced activity of methylentetrahydrofolate reductase (17). This enzyme is involved in the remethylation of homocysteine (Hcy) to methionine (Met) in the homocysteine–methionine pathway; therefore, reduced activity of the enzyme leads to hyperhomocysteinemia or the elevation of homocysteine in serum or blood (12). Szodoray et al. (13) have recently shown that increased serum levels of Hcy is correlated with ocular disease. In addition, the association of the C677T polymorphism with preeclampsia, congenital heart diseases (CHDs), and neural tube defects (NTDs) have been demonstrated (14). Inanir et al. (16) suggested that there is no significant association between genotypic frequencies of the MTHFR C677T polymorphism with risk of RA, but allele frequencies showed statistically significant association (P = 0.01) between patients and controls. In addition, Brambila-Tapia et al. (18) demonstrated that the MTHFR C677T and A1298C polymorphisms in Mexican patients are significantly associated with RA risk.
We must study additional cases in order to verify the accuracy of these results. The association shown here is limited to Khuzestan samples without a separation of the populations. Additional studies that include a comparison with global results and separation of population analysis are needed for approve of results.