1. Background
Spinal cord injury (SCI) is a severe condition, which is characterized by a complex of motor, sensory and neurological dysfunctions, which induces inflammatory responses at the lesion site. These responses attract inflammatory cells to the damaged area and lead to the increase of chemokines in the plasma (1). Membranous CXC Chemokine receptor 4 (CXCR4) and its ligand, stromal-derived factor-1 (SDF-1, namely CXCL12), are members of the chemokine family, which have a critical role in embryo development, immune responses, inflammatory reactions and hematopoietic system modulation (2, 3). Additionally, SDF-1 is a chemokine, which mediates development of neural tissues and early spinal cord progenitor cell differentiation (4). There is good evidence that the administration of growth factors, such as SDF-1a, can stimulate stem cells to repopulate damaged CNS regions (5). Stromal-derived factor-1 is fundamental to central nervous system development, homeostasis and traumatic injury (6). The SDF-1/CXCR4 axis plays an important role in the mobilization of progenitor stem cells during embryogenesis and tissue regeneration (7). Also evidence has indicated that SDF-1/CXCR4 axis plays a crucial role in the recruitment of bone mesenchymal stem cells (BMSCs) to lesion sites in animal models (8). There exists preliminary data indicating that SDF-1a might also be involved in the pathophysiology of SCI. It has been reported that the expression of SDF-1a was positively correlated with the lesion grade after SCI (9), and increased significantly in spinal cord lesions (10). It was demonstrated that SDF-1a and its receptor CXCR4, are upregulated in the injured spinal cord (5). Recent studies have indicated that SDF-1a signaling system may be important for regulating the inflammatory response after SCI (11).
2. Objectives
In this study we investigated the regeneration effects of continuous administration of SDF-1a in a spinal cord injury model in rats.
3. Materials and Methods
3.1. Animals and Experimental Design
Twenty adult male Wistar rats (approximately 250 - 300 g) were used in this study; all animal procedures were approved by the ethical guidelines for the care of laboratory animals of the research center of Iran University of Medical Sciences. The rats were kept in standard cages; at 22°C with a 12:12 light/dark cycle, in a controlled environment with free access to food and water. Animals were randomly divided to four groups (n = 5): Sham group, received laminectomies; SCI group, spinal cord injury; SDF-1 group, spinal cord injury with infusion pump for SDF-1; and vehicle group, spinal cord injury with infusion pump for PBS.
3.2. Spinal Cord Injury Procedure
Rats were deeply anesthetized via an intraperitoneal injection of ketamine and xylazine (80/5 mg/kg). The animals were positioned in a prone and surgery was performed under sterile conditions. Skin was shaved and disinfected with povidone iodine solution. Skin incision and blunt dissection of the muscle layers over the area was performed. After removing the dorsal processes of the 9th and 10th thoracic vertebrae, spinal cord compression injury was performed at the region of the 9 - 10th thoracic segment by 50 g weight clips for one minute (12, 13). The animals received postoperative care including Ringer's lactate solution (1 mL, subcutaneously) for seven days and penicillin/streptomycin (intramuscularly, Invitrogen) for three days. Additionally, bladders were massaged twice a day until bladder reflex returned. Bromodeoxyuridine was injected intraperitoneally, 24 hour after surgery (50 mg/kg) for seven days.
3.3. Implantation of Infusion Pump for Intrathecal Application
Prior to SCI, the animals were placed in a stereotaxic unit. After neck hyper flexion, a midline incision was made in the skin of the nuchal crest, then surrounded muscles were carefully dissected and the cistern magna was exposed, by making 2 mm incision in dura, and a catheter (Spectranetics, USA) was inserted in the incision (at the first cervical vertebra). The incision was subsequently sutured. Seven days after the lesion, for continuous intrathecal SDF-1 administration, an infusion pump (Model 2004, Alzet, USA) was implanted subcutaneously in the neck region filled with 500 ng/mL of SDF-1 dissolved in saline with flow rate of 0.25 m/hour and connected to the catheter (5).
3.4. Tissue Preparation and Immunohistochemistry (BrdU Labeling)
At the end of the experiment, the animals were deeply anesthetized with ketamine and xylazine and were perfused transcardially with 150 - 200 mL PBS followed by 100 mL paraformaldehyde 4%. The spinal cord was dissected and maintained 48 hours in paraformaldehyde 4%. Fixed samples were kept to histological processing and finally tissue specimens were embedded in paraffin. To identify the proliferating cells, the spinal cord sections (5 µm) were treated with 2N HCl at 37°C for one hour and neutralized twice with 0.1 M borate, pH 8.5 for 10 minutes. The sections were then washed three times with washing solution (PBS with 0.2% TritonX-100) and incubated in the blocking solution (PBS containing 3% normal goat serum and 0.2% TritonX-100) for 30 minutes at room temperature. After rinsing, the sections were incubated overnight at 4°C with primary antibody (rat anti-Brdu). The sections were washed with the washing solution and incubated with the corresponding secondary antibody for two hours at room temperature and were mounted. The Brdu positive cells were counted manually after the section was digitally recorded using a microscope (Olympus AX70), Olympus DP11 microscope digital camera and OLYSIA autobioreport software (Olympus optical Co. Ltd. Japan). Cell numbers are expressed as mm2 (14).
3.5. Real Time Reverse Transcriptase-Polymerase Chain Reaction
For analysis of CXCR4 mRNA level, total mRNA was isolated from cells with Trizol reagent (Invitrogen), according to the manufacturer’s instructions. mRNA was reverse-transcribed with a cDNA synthesis kit (Roche, USA). The CXCR4 mRNA levels were determined by Real-Time polymerase chain reaction (RT-PCR), using an ABI PRISMs 7000 Sequence Detection System (ABI, USA). Twenty-five microliter reaction mixtures containing 12.5 µL SYBR Green PCR master mix and 10 ng of cDNA template and primers (5’- CCTCGGGGCCAAATTCAAGA -3’ forward and 5’- GAAGAGTGTCCACCCCGTTT -3’ reverse) for CXCR4 was used. The threshold cycle (Ct), i.e. the cycle number at which the amount of amplified gene of interest reached a fixed threshold, was subsequently determined. Relative quantification of CXCR4 mRNA expression was calculated with the comparative Ct method (15).
3.6. Locomotor Function
Basso, Beattie and Bresnahan (BBB) locomotion rating scale, is the most commonly used test of locomotor function in spinal cord injured rats. Briefly, the rats were placed in an open field box, and the dual hind limb motor function was quantified using a scale ranging from 0 to 21, where 0 corresponds to no locomotor activity and 21 corresponds to normal performance. The test was carried out one day before and after the surgery, and then weekly tests were performed for five weeks to analyze locomotor recovery of spinal cord injury after transplantation (16).
3.7. Statistical Analysis
One-way analysis of variance (ANOVA) followed by post hoc Tukey test was used to determine statistical differences between the experimental groups. Data were expressed as mean ± standard deviation (SD).
4. Results
4.1. Effect of SDF-1 Treatment on Cell Proliferation in Spinal Cord Injury
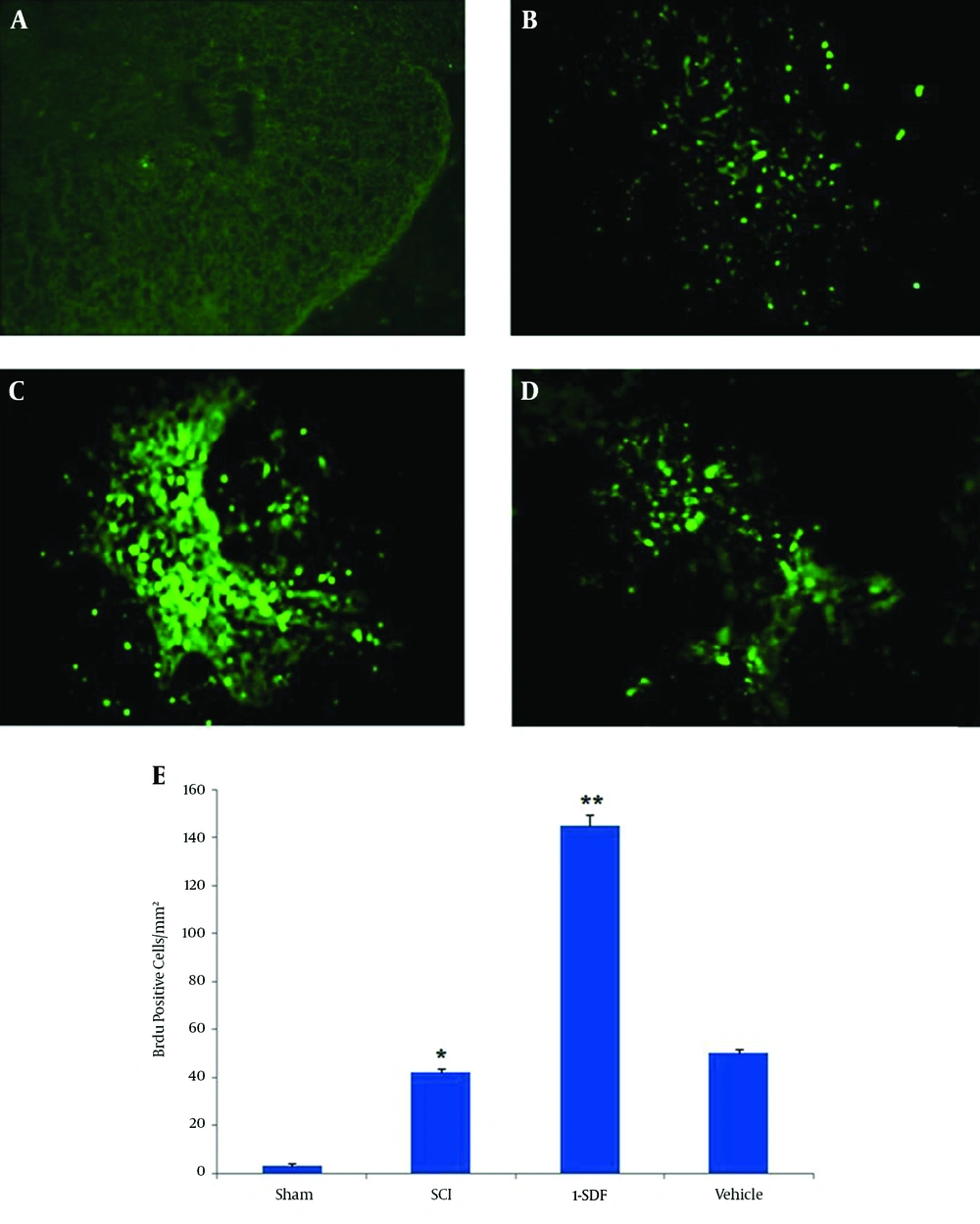
The BrdU positive cells were absent in the spinal cord of sham operated animals, whereas many proliferating cells could be observed after spinal cord contusion injury in other groups (Figure 1). Interestingly, numbers of BrdU positive cells were significantly higher in the SDF-1a group. No difference was observed in the number of BrdU positive cells between vehicle and SCI groups in the contusion regions (Figure 1E).
A, Sham group; B, SCI group; C, SDF-1 group; D, vehicle group (10 ×); E, the graph shows proliferative cell count in all groups. The Brdu positive cells in the SDF-1 group were significantly more than the SCI and vehicle groups. Data are expressed as mean ± SD; *P < 0.001 compare to Sham group; ** P < 0.001 compare to SCI and vehicle group.
4.2. Real Time Polymerase Chain Reaction
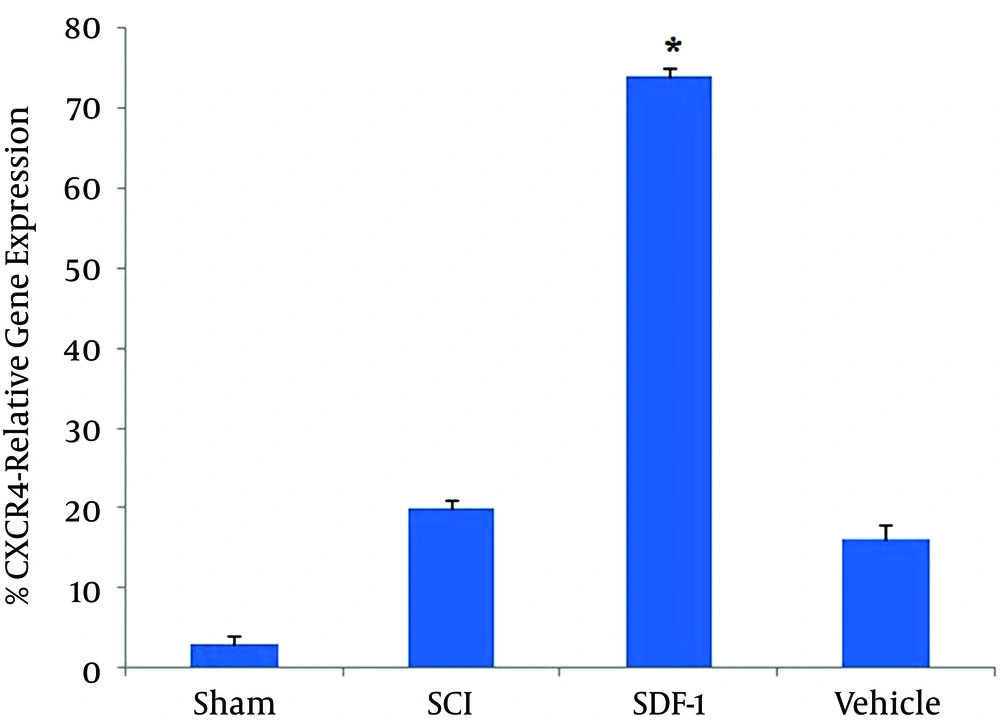
Result showed that the expression of CXCR4 in the SDF-1 group was significantly more than other groups (P < 0.001). No difference was observed in the expression of CXCR4 between SCI and vehicle groups (P > 0.05) (Figure 2).
4.3. Locomotor Function
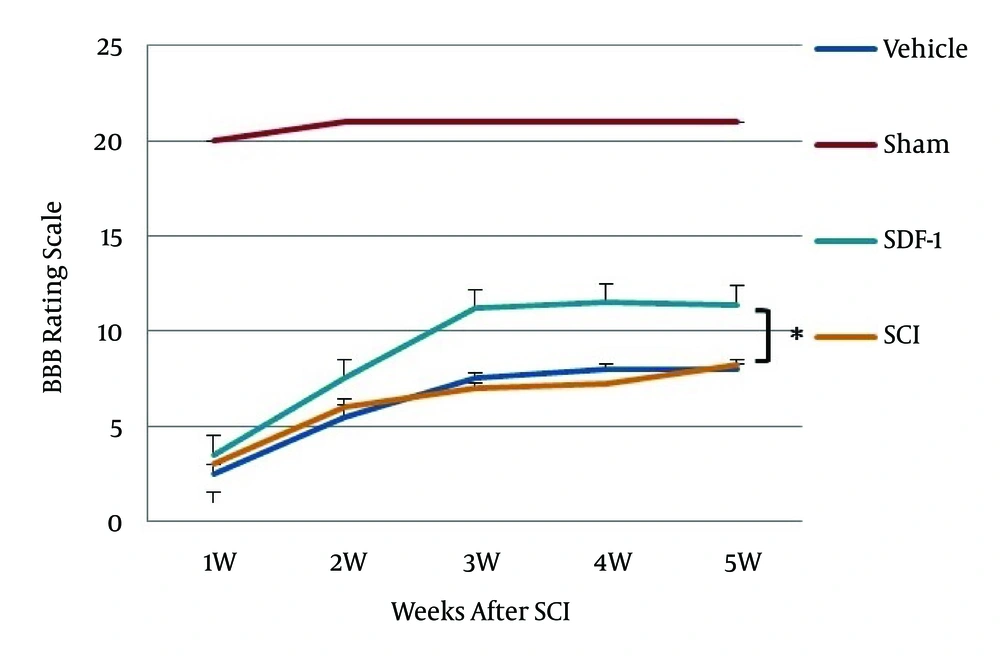
The BBB rating scale was performed within five weeks after surgery. There was no significant difference in BBB score during the first week between injured groups but as shown in Figure 3, after the third week BBB rating scale in the SDF-1 group was significantly higher (10-12) than the SCI and vehicle groups (P < 0.05). These animals were capable of active motility, which revealed that the rats could support weight walking and that forelimb and hind limb coordination ability was good. However, the SCI and vehicle groups achieved a mean BBB score of eight.
5. Discussion
The results of the present study provide evidence that intrathecal SDF-1 improves movement and increases proliferation of cells in the SCI region in rats.
Stromal Cell-Derived Factor-1 is one member of the chemokine family that causes movement and migration of cells from bone marrow via CXCR4 (8, 17-19). The SDF1, after binding to CXCR4, regulates some biological activity in CXCR4-positive cells such as: cell growth, proliferation and angiogenesis (6). Transgenic mice with mutations in SDF1 and CXCR4 gene phenotypes had some abnormalities in their central nervous system and heart (20). Stromal Cell-Derived Factor-1 is secreted from many tissues of the body and is found in organs such as the heart, kidneys, brain, liver, spleen and lungs (21, 22). In CNS, this chemokine is released from microglia, astrocyte and neurons (23, 24). Following CNS injury, the rising of SDF-1 levels is mostly associated with astrocytes and attracts immune and stem cells towards the injury site (6, 25).
There is a physiological dose of SDF-1 in CNS yet in pathologic conditions such as ischemia or injury, glial cells are activated and a large amount is secreted (26).
Furthermore, CXCR4 is expressed in many types of body cells such as lymphocytes, monocytes, neutrophil, epithelial, microglial, endothelial, bone marrow stromal cells, Neural Stem Cells (NSC) and embryonic-like stem cells (27-29). It was demonstrated that SDF1 is involved in migration of NSCs to the injury site of the brain (30-32).
Wang et al. showed that SDF-1α/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model (33).
Hill et al. demonstrated that the expression of SDF-1a and its receptor is increased at sites of neuronal injury (26).
Another study indicated that inhibition of SDF-1 by application of neutralizing antibodies in vivo resulted in a significantly reduced number of human umbilical cord blood cells at the lesion area (34).
The results of real-time PCR of CXCR4 gene in all groups in our study demonstrated that SDF1 attracted CXCR4 cells to the site of the injury; these CXCR4 cells in the SDF-1 group were higher than those in SCI and vehicle groups.
Zededel et al. showed that the number of caspase positive cells decreased and proliferating cells increased dose dependently in the SCI region in rats, which received SDF-1 (5).
In the present study, Brdu-positive cell counting at the spinal cord injury site, number of proliferative cells, increased after injection of SDF-1, which is in agreement with the mentioned studies.
Another finding of our study was the improvement of BBB rating score in SDF-1 group compared to the SCI and vehicle groups, this difference was markedly higher at three to five weeks in the SDF-1 group than the SCI and vehicle groups.
A recent study indicated that erythropoietin mobilizes BMSCs to the lesion site following SCI, enhances the anti-apoptotic effects of BMSCs by up-regulating the expression of the SDF-1/CXCR4 axis and improves neurological outcomes following SCI (8).
In another study, it was shown that chronic administration of SDF-1 1000 mg/mL in SCI rats improved behavioral scores compared to the control group (3). These findings are in agreement with our study. Higher numbers of Brdu-positive cells, CXCR4 expression and higher score of BBB in the SDF-1 group in our study indicated that SDF- 1 have neuroprotective effects in this animal model (35).
This means SDF1 induced migration of CXCR4 cells at the injury site and induced cell proliferation and secretion of some neurotrophic factors that limited damage at the injury site.
5.1. Conclusions
The results of the present study showed that intrathecal administration of SDF-1 increases the CXCR4 cells at the injury site and consequently improves movement in SCI rats, therefore SDF-1 might be a therapeutic option in spinal cord injury, although more research is required in order to elucidate the potential therapeutic role of SDF-1 in SCI recovery.