1. Background
The development of tolerance to morphine analgesia after chronic opioid exposure is a main problem in clinical management of pain that limits effect of the drug to treat chronic pain (1, 2). The molecular mechanism underlying morphine-induced analgesic tolerance is not entirely understood (3-5). Morphine and most clinical analgesic agents act through activation of mu-opioid receptors (6). These receptors are expressed by primary sensory neurons including the small myelinated Aδ-fibers or unmyelinated C fibers as well as neurons at supra-spinal sites of the pain pathway (7, 8). A growing body of evidence shows that desensitization of mu-opioid receptor and the progressive loss of receptor function under continued exposure to the opioid may underlie morphine tolerance (5, 9, 10). However, other molecular changes in other neurotransmitter receptors such as N-methyl-D-aspartate (NMDA) receptors and signaling molecules including protein kinases are proposed to be involved in morphine-induced analgesic tolerance (9, 11-13).
Calcium/calmodulin-dependent protein kinase type II (CamKII) is a serine/threonine kinase that constitutes a family of multifunctional protein kinases and plays a major role in Ca2+-mediated signal transduction (14). The CaMKII is encoded by four genes in mammals known as α, β, γ, and δ (15, 16). All four genes are found in brain (11), but CamKIIα is a neuron-specific isoform (17, 18), which its gene is located on chromosome five in human and on chromosome eighteen in rat (16). CaMKII monomers form oligomers of up to 12 subunits to make a complete enzyme (19). Each monomer is composed of catalytic, variable and oligomerization (also known as association) domains. Once activated, CaMKII phosphorylates numerous target proteins and is involved in many cellular functions, including synaptic plasticity, synaptic vesicle mobilization, regulation of gene expression and modulation of ion channel function (14, 20).
Receptor phosphorylation is thought to be a key initial event for acute mu-opioid receptor desensitization (9). It is shown that several kinases can phosphorylate mu-opioid receptors in response to agonist activation and modulate their activity (21). Limited evidence also shows that CaMKII may modulate function of mu-opioid receptors by phosphorylation (5, 21); therefore, it may be involved in development of morphine-induced analgesic tolerance (5, 22, 23). It is shown that morphine induces a significant up-regulation of spinal and supra-spinal CaMKII activity in tolerant mice (24). In line with recent reports, previous studies showed that administration of CaMKII inhibitors can effectively reverse the development of opioid antinociceptive tolerance and dependence (25-27).
Significant clinical challenges with using morphine arise from tolerance to analgesic effect of the drug. A more effective pain treatment can be achieved when underlying mechanisms of tolerance are recognized and managed (1). According to the above cited data, it is logical to hypothesize that CaMKII is involved in the development of morphine analgesic tolerance and dependence. According to previous researches, changes in translation rates or stability of the existing mRNAs, or modification of the existing proteins may be also critical to induce tolerance and dependence to drugs (28). However, with the knowledge of changes in gene expression it is possible to identify interesting genes and their products involved in morphine-induced analgesic tolerance. Changes in gene expression of CamKIIα in different brain areas during induction of morphine analgesic tolerance were not well studied. The lumbosacral region of the spinal cord is the main region to receive pain signals from hind limbs of laboratory animals. The midbrain also contains regions including periaqueductal gray (PAG) which is a network that modulates nociceptive transmission and is involved in modulation of pain (29, 30). Stimulation of the PAG produces analgesia through the action of endogenous opioids on mu-opioid receptors located in the PAG (29).
2. Objectives
The current study aimed to examine gene expression profile of CamKIIα at mRNA level in the lumbosacral portion of the spinal cord and midbrain during induction of morphine analgesic tolerance in rat.
3. Materials and Methods
3.1. Animals
Male Wistar rats weighing 250 - 300 g were used. The animals were housed in groups of four per each cage and kept in an animal house at a constant temperature (22 ± 2°C) under 12-hours light/dark cycle (light on at 7:00 a.m.). The animals had free access to food and water except during experiments. All animals were handled for 5 minute/day during five days of an adaptation period before the onset of experiments. All experiments were carried out during the light phase from 8:00 to 12:00 (a.m.). A randomized controlled trial study (control and intervention groups) was conducted. Experimental groups consisted of either six rats in behavioral tests or four rats in groups used to evaluate gene expression (31-33). All procedures were performed in accordance with the institutional guidelines for the care and use of laboratory animals in compliance with the guideline for the care and use of laboratory animals (2011), prepared by the national academy of sciences’ institute for laboratory animal research. This project was conducted at department of biological sciences, University of Kurdistan, Iran, in 2015.
3.2. Drugs
Morphine sulfate was purchased from Temad Co. (Tehran, Iran). The drug was dissolved in 0.9% saline and injected intraperitoneally (i.p.) at a volume of 1 mL/kg. Control groups only received saline 1 mL/kg. Those reagents and kits used to evaluate gene expression are described in the appropriate sections in the text.
3.3. Development of Tolerance to Morphine Analgesia
Morphine tolerance was induced in rats by injection of morphine (10 mg/kg) twice-daily (a morning session and an afternoon session on each day) for eight consecutive days. Morphine-induced analgesic tolerance was examined with a hotplate test of analgesia on days one, four and eight of the injections. The hotplate test of analgesia was performed according to the authors’ previous studies (32, 33). In brief, baseline and test latency were recorded for each rat before and after the injection of saline or morphine, respectively. The time elapse between placement of each animal on the hotplate (52 ± 0.1°C) and licking one of the hind paws or first jumping was measured as an index of pain reaction latency. A cutoff time of 80s was set to prevent tissue damage. Finally, the two measured latencies were converted to percentage maximum possible effect (%MPE) using the following formula: %MPE = [(test latency - baseline latency)/ (cut-off time - baseline latency)] × 100.
3.4. Treatments of Experimental Groups to Profile mRNA Level of CamKIIα on Days One, Four and Eight of Morphine Injections in the Lumbosacral Cord and the Midbrain
Six groups of animals were used to evaluate gene expression of CamKIIα during eight days of the repeated injections in the lumbosacral region of the spinal cord and the midbrain. First, two groups received a single injection of saline 1 mL/kg (as control group) or morphine 10 mg/kg (as tolerant group), and after three hours of the injections they were decapitated and submitted to tissue dissection. Second, two other groups of animals received injections of saline 1 mL/kg (as control group) or morphine 10 mg/kg (as tolerant group) twice daily for four days, and three hours after the morning session injection on day four they were decapitated and submitted to tissue extraction. Third, the last two groups of the animals received injections of saline 1 mL/kg (as control group) or morphine 10 mg/kg (as tolerant group) twice daily for eight days and three hours after the morning session injection on day eight they were decapitated and submitted to tissue extraction. After dissection, each tissue was immediately moved to a tube where it was submerged in an RNAlater solution (RNA Stabilization Reagent, Qiagen, USA) followed by overnight incubation at 4°C. After 24 hours, the RNAlater solution was aspirated and the tubes containing the tissues were stored at -70°C until further analysis.
3.5. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from the dissected tissues using the TRIZOL method according to the authors’ previous study (32). Then, total RNAs were analyzed on agarose gel electrophoresis to visualize sharp bands of 28s and 18s ribosomal RNA. The quantification of the total RNAs was also performed using a spectrophotometer (Specord210, Analytic Jena, Germany). Reverse transcription was performed by a revert-aide first strand cDNA synthesis kit (Thermo Scientific, USA). Thermal cycling for amplification of the β-actin (as an internal standard) and the CamKIIα genes consisted of an initial denaturation step (95°C for three minutes) followed by 28 amplification cycles of denaturation (94°C for 30 seconds), annealing (63°C for 30 seconds) and extension (72°C for 30 seconds). A final extension at 72°C for 10 minutes and a termination step at 4°C was also included (C1000 Thermal Cycler, BIO-RAD, USA). “GeneRunner 15” software was used to design primers and the primers were ordered to Takapouzist Company (Tehran, Iran). Some information about primer sequences and product length is summarized in Table 1.
The reaction parameters were firstly adjusted to obtain a condition with a linear relationship between the number of PCR cycles and the initial amount of RNA template relative to PCR products. The PCR products were subsequently analyzed by electrophoresis on 2% agarose gel, and respective bands were quantified by a densitometry method using the Image J software. The densitometry data for the CamKIIα gene expression in each sample was normalized as a ratio of the CamKIIα to the β-actin. Mean of data in control group was set as 100% gene expression and the CamKIIα gene expression data in tolerant group was compared to that of the control group.
| Gene | Accession Number | Primer | Sequence (5’ - 3’) | Start Position | Product Length, bp |
|---|---|---|---|---|---|
| β-Actin | NM_031144.3 | Forward | CTGGGTATGGAATCCTGTGGC | 877 | 201 |
| Reverse | AGGAGGAGCAATGATCTTGATC | 1077 | |||
| CamKIIα | NM_012920 | Forward | TGTGGCGTCATCCTGTATATCTTG | 636 | 391 |
| Reverse | CCTTCACGCCATCATTCTTCTTG | 1026 |
3.6. Statistical Analysis
A two-way repeated measure analysis of variance (ANOVA) was used to analyze the hotplate test data. Further pairwise comparisons were done with post hoc Holm-Sidak test. To analyze the RT-PCR data, the intensity of bands on agarose gel electrophoresis converted to quantitative values using Image J software, and then the results were analyzed with independent sample t-test to investigate differences in the CamKIIα gene expressions between the groups. Values of P < 0.05 were considered statistically significant.
4. Results
4.1. Hotplate Test Revealed That Repeated Injections of Morphine Twice Daily for Eight Days Induced Analgesic Tolerance to the Drug
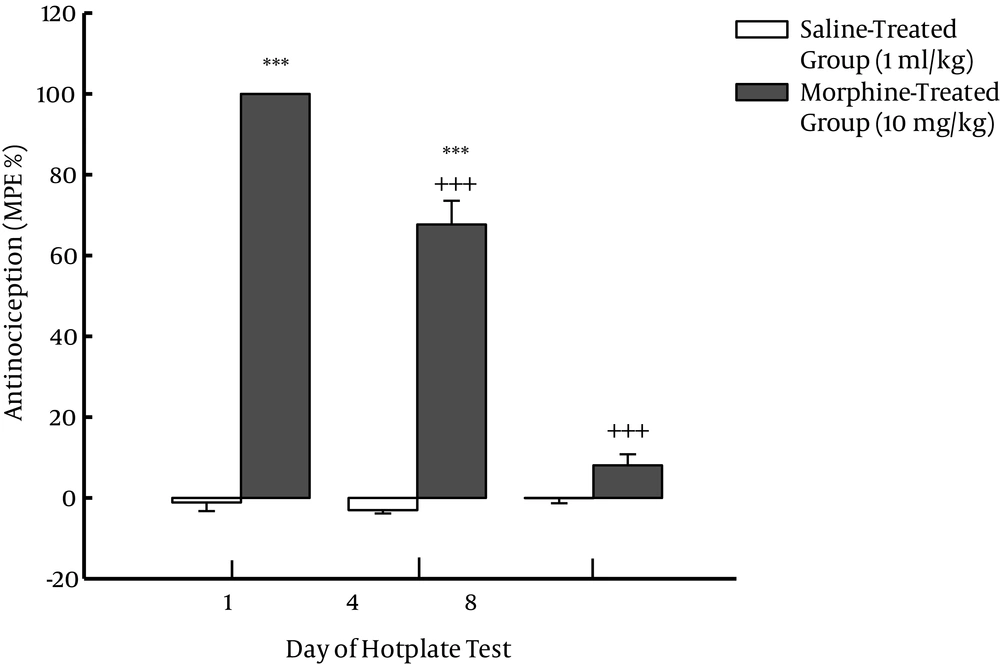
The results of hotplate test were analyzed by a two-way repeated measure ANOVA. Repeated injections of drugs (saline or morphine) were considered as factor A with two levels, and days of the hotplate test were defined as factor B with three levels of days one, four and eight of the injections. The results showed a significant main effect for factor A [F (1, 10) = 571.45, P < 0.001], factor B [F (2, 20) = 140.03, P < 0.001] and interaction of both factors [F (2, 20) = 149.55, P < 0.001]. Post hoc test revealed that morphine at dose of 10 mg/kg on days one and four of the injections induced a significant analgesia but its analgesic effect on day eight was not significant compared to that of saline-treated control group on the respective day. Post hoc test also revealed that analgesic effect of morphine (10 mg/kg) on days four and eight of the repeated injections significantly decreased compared to its effect on day one of the injections (Figure 1).
Two groups of animals were used. One group of the animals received repeated injections of saline for eight days twice daily but the other group received morphine (10 mg/kg) instead of saline. Both groups were tested on a hotplate apparatus on days one, four and eight; first for baseline latency and after 30 minutes of the injections to record test latency. Each bar represents mean ± standard error of the mean (SEM) of MPAE% related to six rats per group. ***P < 0.001 was compared to the control group that received saline on the respective day. +++P < 0.001 was compared to the morphine-treated group on the first day.
4.2. Effect of Morphine Tolerance on mRNA Level of CamKIIα in the Lumbosacral Portion of the Spinal Cord During Eight Days Induction of Morphine Analgesic Tolerance
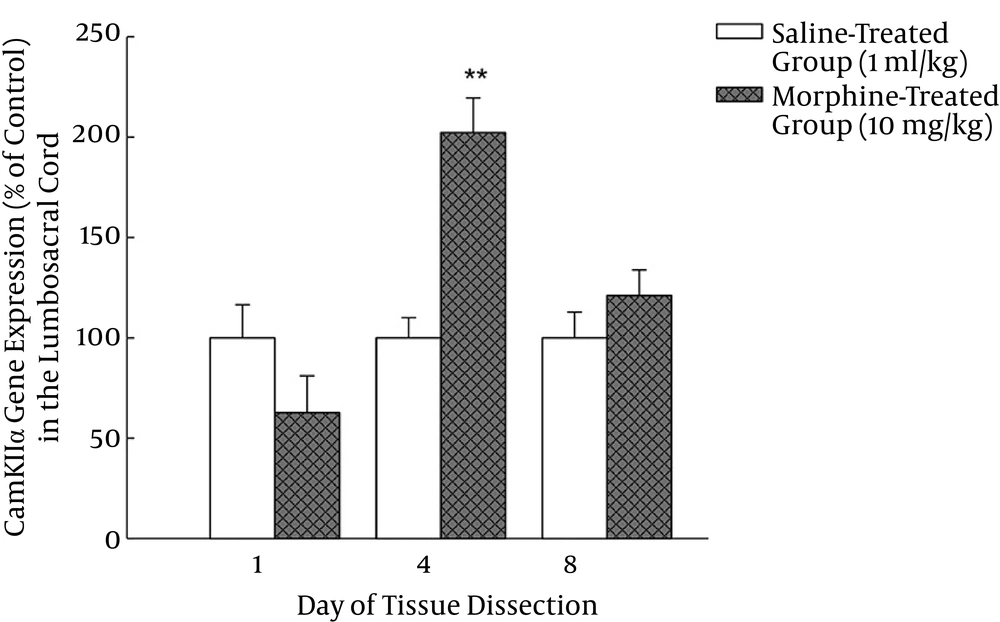
Analysis of the gene expression results by independent sample T-test revealed that the CamKIIα gene expression in the lumbosacral cord in morphine-treated group did not alter significantly on day one of the injections (P > 0.05), but it increased on day four of the injections compared to that of the saline-treated group (P < 0.01) (Figure 2). Furthermore, the CamKIIα gene expression in morphine-treated group did not alter significantly (P > 0.05) compared to the saline-treated group on day eight of the injections (Figure 3).
Six groups of rats (n = 4) were divided into three sets of two groups. Control groups in each set received saline (1 mL/kg) and tolerant groups received morphine (10 mg/kg). The lumbosacral cord was dissected in different sets of animals on days one, four or eight of the injections. Each bar represents mean ± SEM related to the normalized data of the CamKIIα gene expression in four rats per group. **P < 0.01 was compared to the respective saline-treated control group.
4.3. Effect of Morphine Tolerance on mRNA Level of CamKIIα in the Midbrain in Eight Days Induction of Morphine Analgesic Tolerance
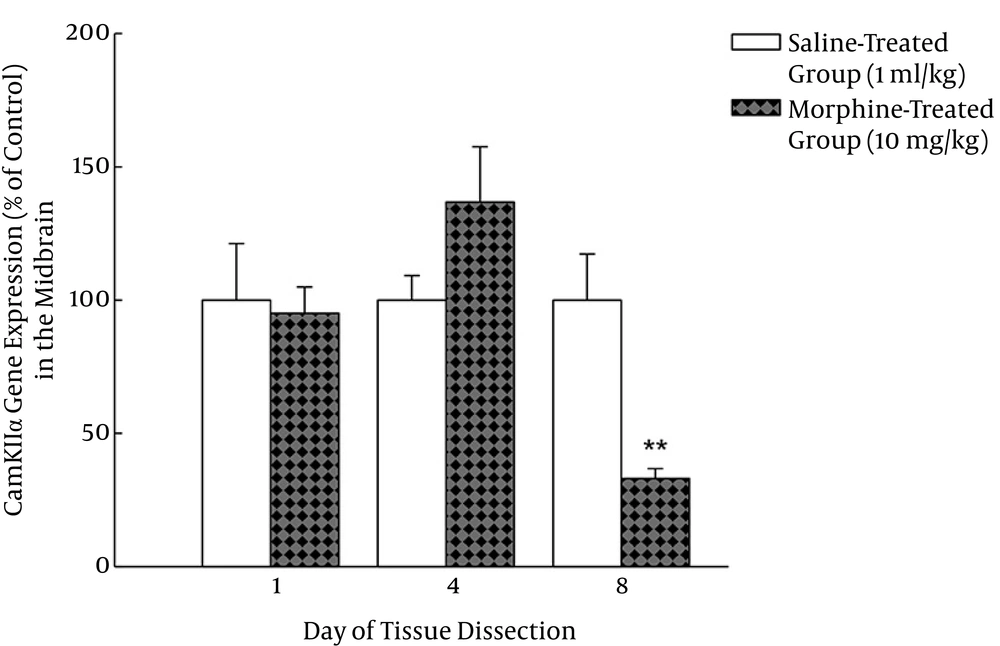
Analysis of the results by independent sample t-test revealed that the CamKIIα gene expression in the midbrain of morphine-treated group did not significantly alter on days one and four of the injections (P > 0.05) but it significantly decreased in morphine-treated group compared to that of the saline-treated group on day eight of the injection (P < 0.01) (Figures 4 and 5).
The midbrain was also extracted on days one, four and eight of the injections from the same animals used for the lumbosacral dissection. Each bar represents mean±SEM related to the normalized data of the CamKIIα gene expression in four rats per group. **P < 0.01 was compared to the respective saline-treated control group.
5. Discussion
The results of the current study showed that twice-daily injections of morphine for eight days induced analgesic tolerance to the drug as revealed by a decrease in morphine-induced anti-nociception on days four and eight of the injections in a hotplate test. There are many reports on the development of tolerance to analgesic effect of morphine after chronic injection of the drug with different routes of administration (31, 34, 35). Different mechanisms such as receptor phosphorylation and desensitization are proposed as underlying mechanisms of morphine tolerance (1, 9, 10). In addition, some protein kinases including CaMKII are involved in morphine tolerance. Wang et al. reported that acute spinal microinjection of KN-93, a CamKII inhibitor, reverses the already-established anti-nociceptive tolerance in rats. They suggested that activation of CaMKII can directly promote opioid tolerance (25). Fan et al. also reported that microinjection of specific CaMKII inhibitors, KN-62 and KN-93, into the hippocampal dentate gyrus before each morphine treatment via inhibition of the kinase activity strongly reduce morphine tolerance and dependence (27). These results support the hypothesis that CaMKII may play an important role in the development of morphine-induced analgesic tolerance. The current study examined gene expression profile of CamKIIα at mRNA level in the midbrain and the lumbosacral portion of the spinal cord in eight days induction of tolerance to the analgesic effect of morphine.
According to the current study results, mRNA level of CamKIIα in the lumbosacral cord did not significantly alter after a single morphine injection on day one but it significantly increased on day four and returned to near the control level on day eight of the injections. A possible interpretation of the results of the CamKIIα gene expression on the first day of morphine injection in the lumbosacral cord may reflect that expression of the CamKIIα at mRNA level is not affected by a single injection of the opioid. Chen et al. also reported that single injection of morphine is not enough to induce changes at gene expression level (36). The analgesic effect of morphine is known to be mediated through mu-opioid receptors coupled to inhibitory G-proteins that subsequently inhibit adenylyl cyclase and decrease the conductance of voltage-gated calcium channels (37). According to previous researches, desensitization of mu-opioid receptors due to phosphorylation of specific residues of these receptors in the third intracellular domains may develop tolerance and dependence on morphine (5, 10). Phosphorylation of some proteins such as cAMP response element binding protein (CREB) by activated CaMKII can modulate their functions which is another key element in opioid tolerance (38). Other investigators also reported that the levels of CamKIIα mRNA and protein robustly increased in spinal cord tissue of morphine-treated mice (39). Considering the expression pattern of the CamKIIα gene in the lumbosacral cord on day four of the morphine injections in the present study, it may be suggested that repeated injections of morphine gradually increases CaMKII and subsequently phosphorylate mu-opioid receptors leading to the onset of analgesic tolerance. It is possible that decrease in Ca2+ level as a result of morphine action leads to a decrease in activation of CaMKII in neurons of the lumbosacral cord, which in turn may increase the CamKIIα gene expression as a compensatory mechanism. The time dependent changes in the CamKIIα gene expression in the lumbosacral cord may be due to neuronal compensatory responses that may occur after an increased expression level of the kinase. It can be suggested that CaMKII in the spinal cord is a key molecule to induce morphine analgesic tolerance but not to maintain this process because its gene expression was returned to near control level on day eight of the injections. Either some other molecules in the spinal cord may underlie maintaining morphine analgesic tolerance or some other mechanisms at supra-spinal sites may be involved. Liang et al. quantified expressions of the CaMKII at mRNA and protein levels and activated phosphokinase levels, and they reported significant increases in these molecules in the lumbar region of the spinal cords of morphine tolerant mice (39). Wang et al. also reported that morphine treatment (15 μg/day for seven days) leads to enhancement of different kinases including p38 as a mitogen-activated protein kinase and CaMKII phosphorylation and activation in the spinal cord dorsal horn (34). Taken together, these results support the vulnerability of the CamKIIα gene expression during induction of morphine tolerance.
The current study results also revealed that the CamKIIα gene expression in rat midbrain on days one and four of the morphine injections did not alter significantly; however, it significantly decreased on day eight of the injections. Considering this decrease in the CamKIIα gene expression in the midbrain, it may be proposed that the CamKIIα gene expression in the midbrain is affected by a latency compared to that of the lumbosacral cord. The CaMKII is a central regulator of long-term synaptic plasticity, learning and drug addiction (40). It is reported that CaMKII negatively mediates mu-opioid phosphorylation and internalization in the midbrain (41). Mu-opioid and NMDA receptors associate in the postsynaptic structures of PAG neurons. Morphine disrupts this complex by protein kinase-C (PKC)-mediated phosphorylation of NMDA receptors and therefore potentiates the NMDAR-CaMKII pathway implicated in morphine tolerance (29). One explanation for the decrease in the CamKIIα gene expression in the midbrain is that repeated use of morphine increases calcium entry via NMDA receptors into the neurons leading to more activation of CamKII in the midbrain and finally sending inhibitory signals to nucleus to decrease its gene expression. A region-specific pattern of the CamKIIα gene expression is reported by other investigators in different brain areas after morphine treatment (36). However, there are no direct reports examining the CamKIIα gene expression in the midbrain during induction of morphine tolerance. According to what was described, CamKIIα in the midbrain neurons may play a direct and central role in opioid tolerance. The current study suggested a specific association between the CamKIIα gene expression in the lumbosacral cord and midbrain with induction of morphine tolerance. Different patterns of the CamKIIα gene expression in the lumbosacral cord and midbrain reflect different responsibilities of the two sites for repeated injections of morphine and induction of analgesic tolerance to morphine.
5.1. Implication of These Results for Research
Gene expression of CamKIIα in the lumbosacral cord and midbrain were differently affected in eight days of induction of morphine tolerance with a site-specific pattern including an increase in the spinal cord and a decrease in the midbrain with a specific time pattern. Changes in the CamKIIα gene expression responding morphine injection were more sensitive in the spinal cord compared with the midbrain. These results further support the involvement of the CamKIIα in induction of morphine tolerance.