1. Background
Mesenchymal stem cells (MSCs) are multi-potent cells, which can differentiate into mesenchymal and trans-differentiate to non-mesenchymal tissues in vitro and in vivo (1). They are found in specialized tissues of the body like the brain, bone marrow, Wharton jelly, pulp and umbilical cord blood (2). Stem cells and especially MSCs produce all types of multicellular tissues in the body via proliferation and differentiation in controlled in vivo environments. These cells are characterized by their easy access, expanding potential in vitro and features that are not easily lost (3). Bone-marrow mesenchymal stem cells (BMSCs) are non-hematopoietic stromal cells with powerful proliferative and differential potential and represent interesting modalities for the establishment of efficient stem cell-based therapy in various diseases. In the recent years, there has been growing interest in MSC application in cell-based therapy (4-6). However, MSCs-based therapy faces some limitations such as low proliferation and survival rate (7-9) after replacement in an in vivo environment (10-12). Following MSCs transplantation, the cells are faced with factors such as low serum, hypoxia, heat shock, and oxygen free radicals. This environment-induced cell death and apoptosis hours after transplantation (13). There are several strategies to improve survival rate, potency, migration and targeting of MSCs. Some studies have reported that preconditioning of MSCs by cultivation under H2O2, hypoxic and serum deprivation as well as sub-lethal stress environment improves the survival and proliferation rates, differentiation and migration capacities (14, 15). Preconditioning using H2O2 is the most practical and important protocol applied in preclinical studies. It has been suggested that preconditioning exerts its effects through altering intrinsic signaling pathways of growth factors, cytokines, and chemokines and expression level of their receptors and consequently improves stem cells survival and proliferation rate (16, 17). Treatment of MSCs with low concentrations of H2O2 for a short period of time adapts them to harmful conditions. It protects MSCs against oxidative damage upon their exposure to high concentrations of reactive oxygen species (ROS) and prevents apoptosis induction as well (18). Enhancement of heart function and decrease of myocardial fibrosis have been reported following transplantation of H2O2 preconditioned MSCs to infracted myocardium in mice (19). Interestingly, H2O2 preconditioning decreases serum deprivation (SD)-induced apoptosis.
2. Objectives
In the present study, protective properties of co-preconditioning of MSCs with low serum and H2O2 were investigated in in vitro.
3. Methods
3.1. Mesenchymal Cells Isolation and Culture
Mesenchymal stem cells (MSCs) were obtained from the femoral and tibial bones of six-week-old Wistar Albino male rats. These cells were suspended in low glucose Dulbecco’s modified eagle medium (DMEM) medium with 10% fetal bovine serum (FBS) (Gibco, Invitrogen, CA, USA) and incubated at 37°C in a humidified chamber with 5% CO2. The culture medium was completely replaced every three days and non-adherent cells were removed. Mesenchymal Stem Cells were recognized by their capability to proliferate in culture with an attached well-spread morphology. Once cells were more than 80% confluent, adherent cells were trypsinized and re-plated 1: 3 (passage1). The differentiation potency of MSC was confirmed by induction of osteogenic, chondrogenic and adipogenic differentiation using specific media.
3.2. Differentiation of Bone Marrow Mesenchymal Stem Cells (BMMSC)
The multi-potency of bone marrow mesenchymal stem cells (BMMSC) was confirmed by induction of osteogenic, chondrogenic and adipogenic differentiation using specific differentiation media.
3.3. Osteogenic Differentiation
For osteogenic differentiation, BMMSCs were seeded at density of 2 × 104 cells/cm2 to a 24-well plate and then induced to differentiate in an osteogenic induction medium composed of DMEM with 10% FBS (Gibco, Invitrogen, CA, USA), 100 units/mL penicillin and 100 g/mL streptomycin, 10 nM dexamethasone, 50 µg/mL ascorbic acid, 10 mM -glycerophosphate. The cells were incubated for 21 days in 5% CO2 at 37°C. After 21 days, osteoblast differentiation was evaluated by 2% Alizarin Red (Sigma) staining. Briefly, Cells were washed three times with Phosphate Buffer Saline (PBS) (Gibco, Invitrogen, CA, USA) and then fixed for 15-30 minutes with 4% formaldehyde. After rinsing three times with distilled water, they were stained with Alizarin red for three minutes. The cells were washed with distilled water several times and examined under the microscope.
3.4. Adipogenic Differentiation
The cells were seeded at a density of 2 × 104 cells/cm2 in 24-well plates. After 80% confluency, the cells were exposed to adipogenic induction medium, consisting of DMEM supplemented with 10% FBS, 100 units/mL penicillin and 100 g/mL streptomycin, 100 nM dexamethasone, 50 µg/mL indomethacin for 10 days and fat droplets were examined under the microscope.
3.5. Chondrogenic Differentiation
The cells were cultured at a density of 2 × 104 cells/cm2 in 24-well plates. Chondrogenic induction medium was added to cells for 21 days. Medium was changed twice a week. After 21 days, the cells were stained with Alcian blue (Sigma). Briefly, the wells were washed with PBS and fixed with 4% paraformaldehyde for 15 - 30 minutes. Then, the cells were stained with Alcian blue for 30 minutes and washed with HCL 0/1 N. The plate was examined under a light microscope.
3.6. Preconditioning of Mesenchymal Stem Cells
The cells were cultured in 96-well plates at concentration of 104 cells/well. The experiment was designed with six groups. The control group received DMEM with 10% FBS, group I received 5 μM H2O2, group II received 10 μM H2O2, group IV received 0.5% serum and group V received 5 μM H2O2 + 0.5% serum. All groups were cultured and incubated for 6, 12, 24 and 48 hours in 37°C with 5% CO2. Then, all groups were exposed to a lethal dose of 300 μM of H2O2 for 24 hours followed by 24 hours of incubation with fresh medium composed of DMEM and 10% FBS as a recovery period. Finally, MTT assay was performed to detect proliferation of the cells in different groups.
3.7. MTT Assay
Cell proliferation evaluation was confirmed by MTT assay. Briefly, 100 µL of the culture medium was removed and 15 μL MTT 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (5mg/ml) was added. After three hours, the wells were replaced with 200 μL of dimethyl sulfoxide (DMSO). The wells were pipetted and read with a spectrophotometer (ELISA reader, TECAN/ sunrise, Magellan program, Austria) at optical density of λ570. Percentage of viable cells was measured by:

3.8. Statistical Analysis
All results were expressed as mean ± standard deviation. Statistical analyses were performed by one-way analysis of variance (ANOVA) using SPSS software version 20. The differences between means was considered statistically significant when the probability level (P value) was less than 0.05 (P < 0.05).
4. Results
4.1. Morphology of Mesenchymal Stem Cells
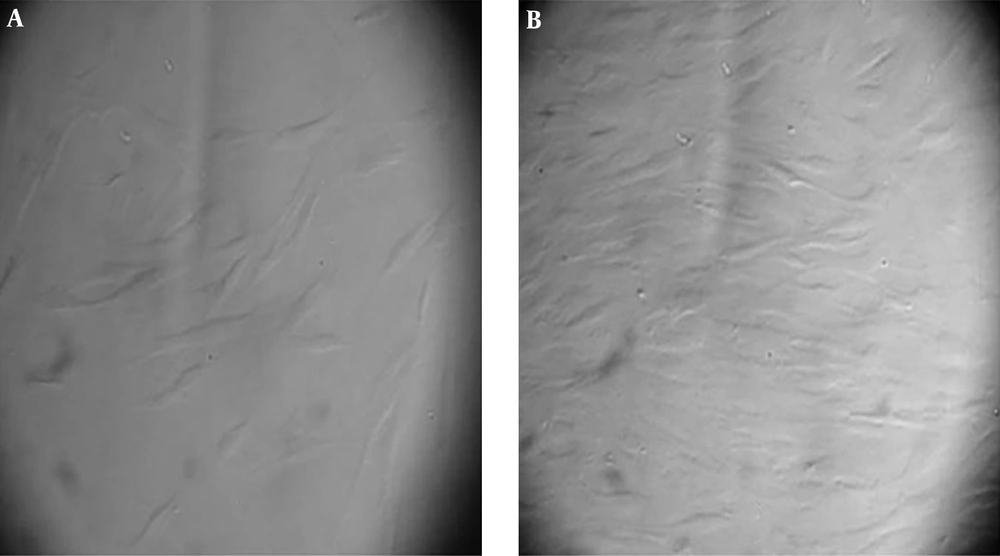
After one week, the attached cells were found in the flasks. The cells were attached to plastic and their morphology was fibroblast like (Figure 1).
4.2. Differentiation of Mesenchymal Stem Cells
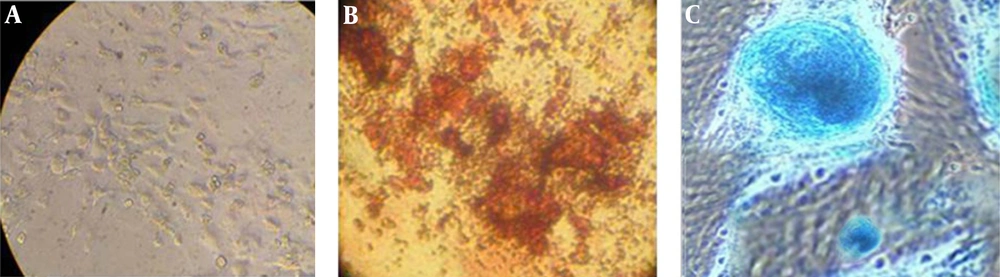
The cells were cultured with differentiation media for 21 days (osteogenesis and chondrogenesis) and 10 days for adipogenesis. As it is illustrated in Figure 2 the MSCs differentiated to bone, adipose and cartilage tissue (Figure 2 A-C).
4.3. Improvement of Cell Proliferation After Preconditioning
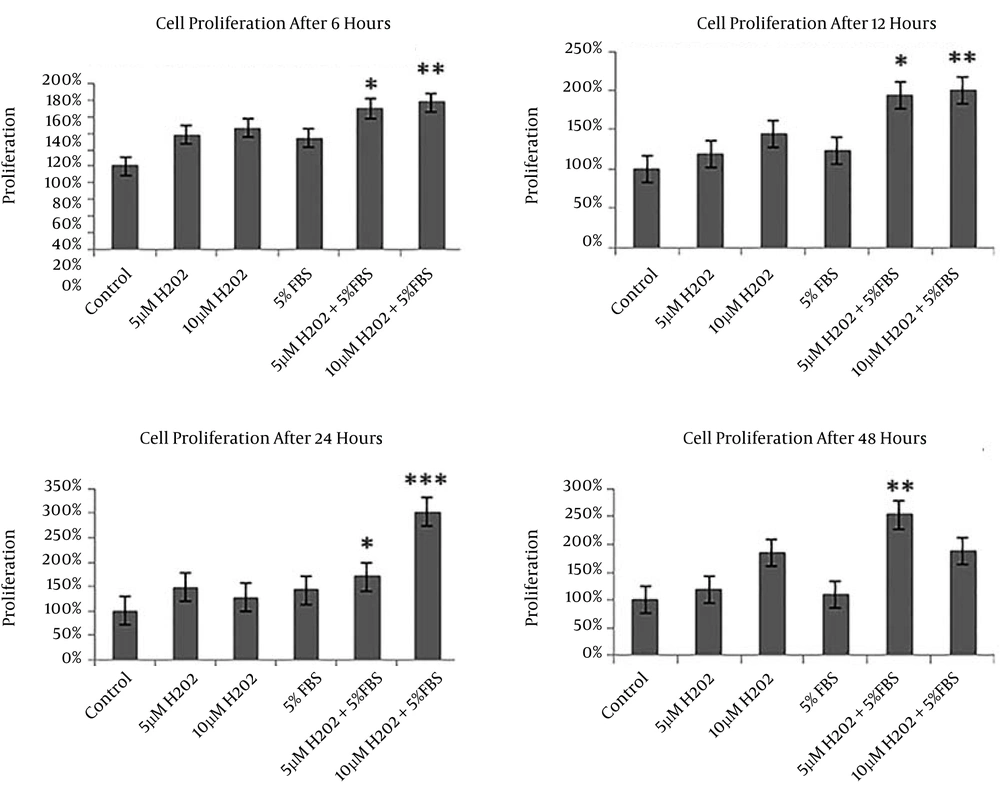
Cell proliferation rate of different groups after preconditioning is shown in Figure 3. It is clear that cell proliferation increased in all preconditioned groups compared to the control. Also, it is indicated that cell proliferation in groups IV (5 μM H2O2 + 5% FBS) and V (10 μM H2O2 + 5% FBS) increased significantly compared to the other groups after 6, 12 and 24 hours of treatment (P < 0.05). Moreover, significant increase in cell proliferation was observed only in group IV (5 μM H2O2+ 5% FBS) towards the other groups after 48 hours (P < 0.05) (Figure 3).
5. Discussion
In spite of its benefits, cell therapy for treatment of different diseases has been limited by poor survival ability of MSCs and their cell death due to in vivo ischemic environment (8, 17, 20), therefore, it is essential to discover a novel strategy to improve the efficiency of MSCs for successful stem cell transplantation. In this regard, the present study investigated the MSCs survival ability following preconditioning with cultivation under hypoxic and serum deprivation conditions. The major finding of this study was the improvement of MSCs proliferation after preconditioning with H2O2 and serum deprivation. Preconditioning with low concentrations of H2O2 and FBS deprivation effectively enhanced the proliferation of MSCs in a dose and time-dependent manner. Several in vitro and in vivo studies reported increase of MSCs survival after preconditioning under hypoxic and serum deprivation conditions (15, 21). Indeed, some studies have shown that rate of successful engraftment and differentiation capacity of the pretreated MSCs was higher than non-preconditioned controls (14, 22). In some studies, higher viability rate and clonogenic capacity was reported after serum deprivation-treated MSCs consequently cultivated in presence of FBS (16, 23). Also, it has been stated that MSCs cultured in serum deprivation conditions show normal morphology (24). However, the detailed mechanisms involved in MSCs protection following preconditioning are not fully determined. It is suggested that, alteration of intrinsic signaling pathways of some growth factors, cytokines, and chemokines and the expression levels of their receptors (especially CXCR4) due to preconditioning might increase their efficiency (25). Preconditioning with H2O2 facilitates stem cell differentiation, heart regeneration, and enhancement of early cardiac development by activating the Notch and Wnt signaling pathways, and it also prevents induction of apoptosis in MSCs (26). Low H2O2 concentration enhances expression of CXCR4 at gene and protein levels and also facilitates the interaction between CXCR4 and its ligand, and stromal cell derived factor-1α (SDF-1α) promotes migration capability of MSCs (27).
In spite of its efficiency in preclinical studies, its application in clinical trials needs more attention in regards to safety of preconditioning.
5.1. Conclusions
Our findings confirmed that preconditioning with H2O2 as well as serum deprivation improves MSCs proliferation ability.