1. Background
Gastric carcinoma (GC) is the fourth most common cancer and the second most common cause of tumor related deaths worldwide (1, 2). Despite its decreasing incidence, GC remains a major health problem (3, 4). So that, approximately 700,000 people die of GC every year (5). About 70% of cancer deaths occur in the developing countries (3). There are variations in GC geographical distribution from place to place and from one generation to another (1). The highest prevalence rates are reported in East Asia, Eastern Europe and South America, and the lowest prevalence in North America and a large part of Africa (3). In Asia, the countries with high prevalence include Japan, China, South Korea and countries with low prevalence include India, Pakistan and Thailand. GC is the most common malignancy in Iran. In the North-West of Iran, the highest prevalence is in Ardabil. In South-East Iran the GC is the second intestinal-gastrointestinal malignancy (6). Its etiology is not fully elucidated yet. Epidemiologic data indicate that environmental factors are important in the pathogenesis of GC (7). These factors include Helicobacter pylori (H. pylori) infection, high salt intake, high consumption of nitrous compounds, cigarette smoking, gastric surgeries, gastric ulcer and blood group A as a predisposing genetic factor. Among these, H. pylori infection is established as a necessary factor in the development of GC (8-10). Although many studies show that H. pylori infection is closely associated with gastric carcinogenesis, the mechanisms and processes of carcinogenesis by H. pylori are largely unknown (11).
Helicobacter pylori are Gram-negative spiral-shaped bacteria and can colonize the gastric mucosa for a long time (12). Following the bacterial colonization and gastric mucosal damage, epithelial cell proliferation increases in response to injury. This event may cause increment of DNA damage, enhancement of malignancy and finally development of GC (13).
Gastric carcinogenesis is a multi-step process, ranging from chronic gastritis to atrophy; loss of gastric glands, intestinal metaplasia (IM), dysplasia (DYS) and ultimately gastric cancer (2, 14).
Chronic gastritis is the inflammation of gastric mucosa. It is characterized by elementary lesions whose extent and distribution are related to their etiology and host responses. Morphological changes in gastric mucosa of gastritis consist of epithelial degeneration, neutrophilic infiltration, mononuclear inflammatory cell infiltration, atrophy, parietal cell alterations and finally IM (15).
IM is defined as replacement of the gastric epithelial mucosa by an epithelium resembling the small intestine (16, 17). IM and DYS are considered as gastric precancerous lesion. They are created as a result of long-term and progressive gastric mucosa damages (18).
DYS is characterized microscopically by a set of histological alterations. The diagnosis relies on cytological parameters such as cytoplasmic mucin depletion, cellular crowding, pleomorphism, stratification, loss of cellular and nuclear polarity, nuclear hyperchromatism, increased nuclear to cytoplasmic (N/C) ratio and increased mitotic activity. Architectural abnormalities comprise glandular disarrangement or budding with irregular branching, dilatation and intraluminal folding. Morphologically, DYS differs from normal gastric mucosa, and it is shown that DYS is associated with a higher risk of gastric carcinogenesis (19).
Several biological markers are tested as potential predictors of the GC outcome and some of them are essential to develop malignancy. The p53 gene is one of the tumor suppressor genes that play a complex role in the cell cycle regulation. Mutation of this gene is very common and associated with carcinogenesis, which results in the loss of gene function (9).
Recently, Shadifar et al. (11) showed that H. pylori infection has a direct role on p53 mutagenesis in patients with IM, DYS and GC. Tumor suppressor gene or p53 produces the P53 protein, which is located in the cell nucleus (2). Increase of P53 protein production is very common in human cancers (20, 21).
The p53 gene activates when DNA is damaged. It leads to cell cycle arrest in G1-phase (2) and repairs the damaged DNA, or cell apoptosis (22). If p53 gene is damaged, it can allow a cell to go from normal into uncontrolled growth, thus leads to carcinogenicity (23). The wild-type P53 protein has a very short half-life of about 5 to 20 minutes and does not accumulate inside cells for a long time (24). The p53 damaged gene can cause production of a protein with long half-life that remains within the cell for a long time. Therefore, it can be detected using immunohistochemistry method (25). It is shown that accumulation of P53 protein increased during the process of gastro carcinogenicity with the progression from gastritis toward IM, DYS and GC (26).
Another marker that is implicated in the progression of cancer is the Ki-67 antigen. At first, it was recognized by Gerdes et al. (cited in Inwald et al. (27)) in the early 1980s. The Ki-67 or Nuclear antigen is related to cell proliferation process. It is expressed at different phases of the cell cycle (14). Cells express the Ki-67 antigen during G1, S, G2, and M phases, but this antigen cannot be observed in the resting phase or G0 phase (14, 28, 29). The expression of Ki-67 increases in many tumors (29). Forones et al. (30) demonstrated that the Ki-67 proliferating index increased during the process of gastro carcinogenicity from IM to GC.
Since GC is a multifactorial disease with significant geographical variations, confused pathogenesis and controversies in the results among different geographic regions determined by numerous environmental and host genetic factors (31), the molecular basis of GC is investigated in numerous studies. The lack of data in the studied geographical region and population and also lack of enough knowledge about the role of H. pylori infection in the process of GC were major motivations behind the current study. Hence, the contribution of this paper is to evaluate the role of H. pylori infection as a gastric cancer risk factor on p53 and Ki-67 immunohistochemical expressions in cases of IM, DYS and GC.
2. Objectives
The current study aimed to evaluate the effects of H. pylori infection on immunohistochemical expression of p53 and Ki-67 in gastric specimens of patients with IM, DYS and GC.
3. Methods
3.1. Study Design
This descriptive-analytic study was conducted on 122 gastric specimens selected from the pathologic files archive of Ali-ebne-Abitaleb hospital, Zahedan University of Medical Sciences (ZAUMS), Zahedan, Iran. All samples had been obtained by endoscopic biopsy from patients admitted from 2010 to 2015. The inclusion criteria of the selection of archival histological specimens were suitable formalin fixed paraffin-embedded tissue from patients with gastric cancer and precancerous lesion and a complete clinicopathological data. The exclusion criteria were the samples from patients with immune disorders, recent recipient of steroids and anti-H. pylori drugs.
The project was approved by the ethics committee of Zahedan University of Medical Sciences (IR.ZAUMS.REC.1394.53).
The study specimens were classified according to the histological diagnosis: 42 IM (20 cases with H. pylori infection and 22 without H. pylori infection), 38 DYS (19 cases with H. pylori infection and 19 without H. pylori infection) and 42 GC samples (19 cases with H. pylori infection and 23 without H. pylori infection).
Selected formalin-fixed paraffin-embedded tissue blocks were cut into three micrometer thick sections mounted on glass slides for histological and immunohistochemical evaluations.
Histological evaluations of the specimens were done based on H&E sections and all selected samples were re-examined by an expert pathologist to ensure prior diagnosis.
3.2. Detection of Helicobacter pylori Infection in Gastric Biopsy Specimens
Helicobacter pylori in the gastric tissue were detected by histological examination; using H&E, modified Giemsa and carbol fuchsin staining methods. Specimens in which all three assays were positive were classified as the group with H. pylori infection specimens and when the results of all three tests were negative, they were considered as group not infected with H. pylori specimens.
3.3. Immunohistochemical Analysis
Immunohistochemical expression of p53 and Ki-67 genes in biopsy samples of patients with IM, DYS and GC were performed using the monoclonal mouse anti-human antibodies p53 (RTU-P53-DO7, Novocastra, England), and Ki-67 (RTU-Ki-67-MMI, Novocastra, England), respectively. Time, temperature and storage conditions were according to the manufacturer’s instructions. The tissue samples were cut into three micrometer sections using the fully automated Leica RM2255 instrument (Leica, Germany) and mounted on HistoGrip (CEDARLANE, Canada) coated slides. The sections were deparaffinized and rehydrated. Then they were transferred into sodium citrate buffer (pH: 6.0) and boiled at 120°C for a total of 20 minutes. After that, the sections were left to cooldown to room temperature for 15 minutes. Immunostaining was precisely described in the authors’ previous study (21).
Positive and negative controls were performed at the same time for each section. The positive controls were respectively appendix tissue for Ki-67, and a specimen of colon cancer; previously shown to express p53 for p53 immunostainings. Negative control was obtained by incubation of slides in Tris-buffered saline (TBS) buffer and omitting the primary antibodies. Slides were evaluated under a light microscope at high-power magnification (X400) and scoring was done by two expert histologists blindly.
3.4. Evaluation of Immunostaining
The intensity of staining for p53 and Ki-67 were scored as follows: 0 for negative; 1 for weak or Mild (yellow or light-brown nuclear stain); 2 for moderate (dark-yellow, medium-brown stain or a mixture of light and dark stain) and 3 for strong (dark-brown stain in most of the nuclei) (10). The extent of staining was classified according to: 0, < 5%; 1, > 5% - 25%; 2, > 25% - 50%; 3, > 50% - 75%; 4, > 75%, and the final score was calculated by multiplying the percentage of positive cells by staining intensity. Generally, the final results were within the range of 0 - 12, scores higher than four were considered positive expression and less than four were considered as negative expression (32).
3.5. Statistical Analysis
Statistical analyses were performed using SPSS ver. 16. Statistical differences between independent groups were assessed by Kruskal-Wallis and Mann-Whitney U tests. All data are presented as the mean ± standard error of the mean (SEM). P < 0.05 were considered significant.
4. Results
4.1. Clinicopathological Data
A total of 122 cases (77 males and 45 females) with the age range of 18 - 92 years were included in this study; 47.54% of the cases had H. pylori infection and 52.45% were without H. pylori infection. Table 1 shows the demographic profile and H. pylori status of the specimens.
| Variable | Intestinal Metaplasia | Dysplasia | Gastric Cancer |
|---|---|---|---|
| Age range, y | 22 - 81 | 25 - 86 | 18 - 92 |
| Gender, No. (%) | |||
| Male | 21 (50) | 33 (86.8) | 23 (54.7) |
| Female | 21 (50) | 5 (13.2) | 19 (45.2) |
| Helicobacter pylori infection | |||
| Positive | 20 (47.61) | 19 (50) | 19 (45.23) |
| Negative | 22 (52.38) | 19 (50) | 23 (54.76) |
| No. (%) | 42 (34.43) | 38 (31.15) | 42 (34.43) |
4.2. The p53 and Ki-67 Expression
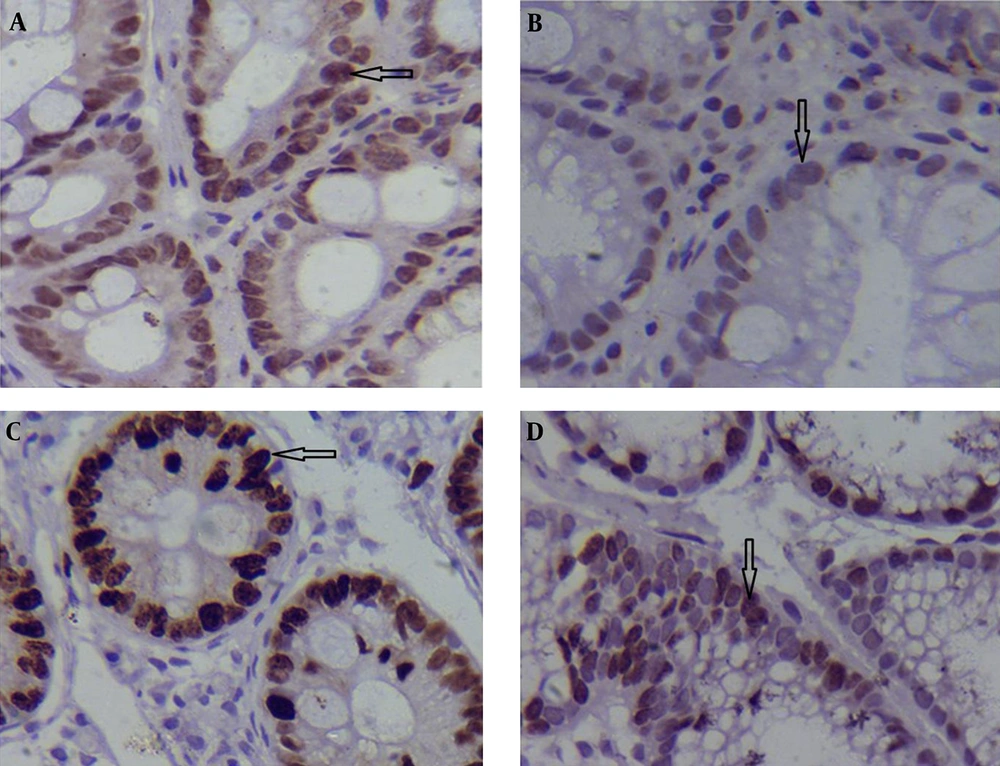
The expression of the p53 and Ki-67 genes was compared between the samples with and without H. pylori infection at different pathological stages (Table 2). Specific staining with both monoclonal anti-p53 and -Ki-67 was exclusively confined to the nuclei of the malignant cells. The study data showed that in patients with IM, average p53 expression (5.70 ± 0.64 for cases with H. pylori infection vs. 3.40 ± 0.51 for those without H. pylori infection; P = 0.013 < 0.05; Table 3); and Ki-67 gene were significantly higher in specimens with H. pylori infection compared to those without H. pylori infection (4.55 ± 0.44 vs. 2.95 ± 0.38; P = 0.014 < 0.05; Table 3). Figure 1A-D shows immunohistochemical staining of p53 and Ki-67 positive cells in the groups including specimens with and without H. pylori infection.
| Group No. (%) | N | Helicobacter pylori | No. Infection | p53 Positive, No. (%) | P Value | Ki-67 Positive, No. (%) | P Value |
|---|---|---|---|---|---|---|---|
| IM | 42 | + | 20 | 12 (60.0) | 0.014 | 11 (55) | 0.005 |
| - | 22 | 5 (22.72) | 3 (13.63) | ||||
| DYS | 38 | + | 19 | 11(57.89) | 0.103 | 5 (26.31) | 0.049 |
| - | 19 | 6 (31.57) | 11 (57.89) | ||||
| GC | 42 | + | 19 | 13(68.42) | 0.014 | 14 (73.68) | 0.207 |
| - | 23 | 7(30.43) | 14 (60.86) |
Abbreviations: IM, intestinal metaplasia; DYS, dysplasia; GC, gastric cancer.
| Group No. (%) | N | Helicobacter pylori | No. Infection | p53 Positive, ( Mean ± SEM) | P Value | Ki-67 Positive, ( Mean ± SEM) | P Value |
|---|---|---|---|---|---|---|---|
| IM | 42 | + | 20 | 5.70 ± 0.64 | 0.013 | 4.55 ± 0.44 | 0.014 |
| - | 22 | 3.40 ± 0.51 | 2.95 ± 0.38 | ||||
| DYS | 38 | + | 19 | 6.52 ± 0.60 | 0.011 | 3.47 ± 0.47 | 0.027 |
| - | 19 | 4.47 ± 0.25 | 5.21 ± 0.51 | ||||
| GC | 42 | + | 19 | 7.89 ± 0.85 | 0.001 | 6.73 ± 0.60 | 0.097 |
| - | 23 | 3.65 ± 0.59 | 4.95 ± 0.73 |
Abbreviations: IM, intestinal metaplasia; DYS, dysplasia; GC, gastric cancer; N, number of cases in each group.
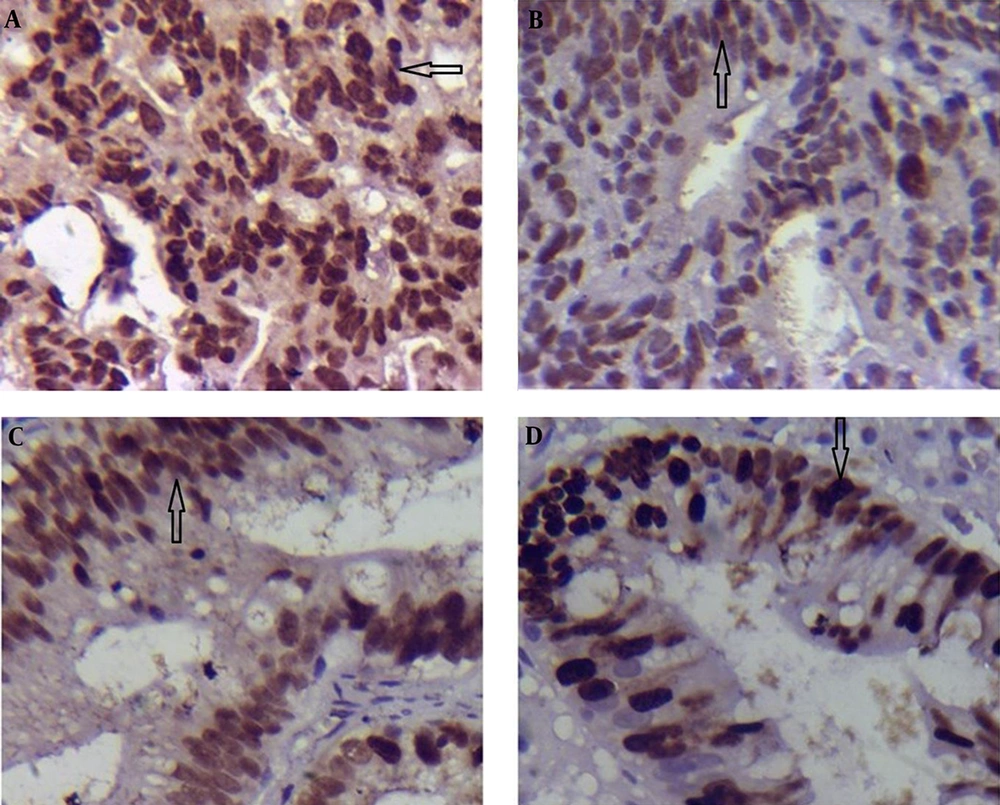
In DYS, only p53 expression was significantly higher in the group with H. pylori infected compared to that of the group without H. pylori infected (6.52 ± 0.60 vs. 4.47 ± 0.25; P = 0.011 < 0.05; Table 3; Figure 2A and B); while the expression of Ki-67 in the tissues infected with H. pylori was lower than that of the specimens not infected with H. pylori (3.47 ± 0.47 vs. 5.21 ± 0.51; P = 0.027 < 0.05; Table 3; Figure 2C and D).
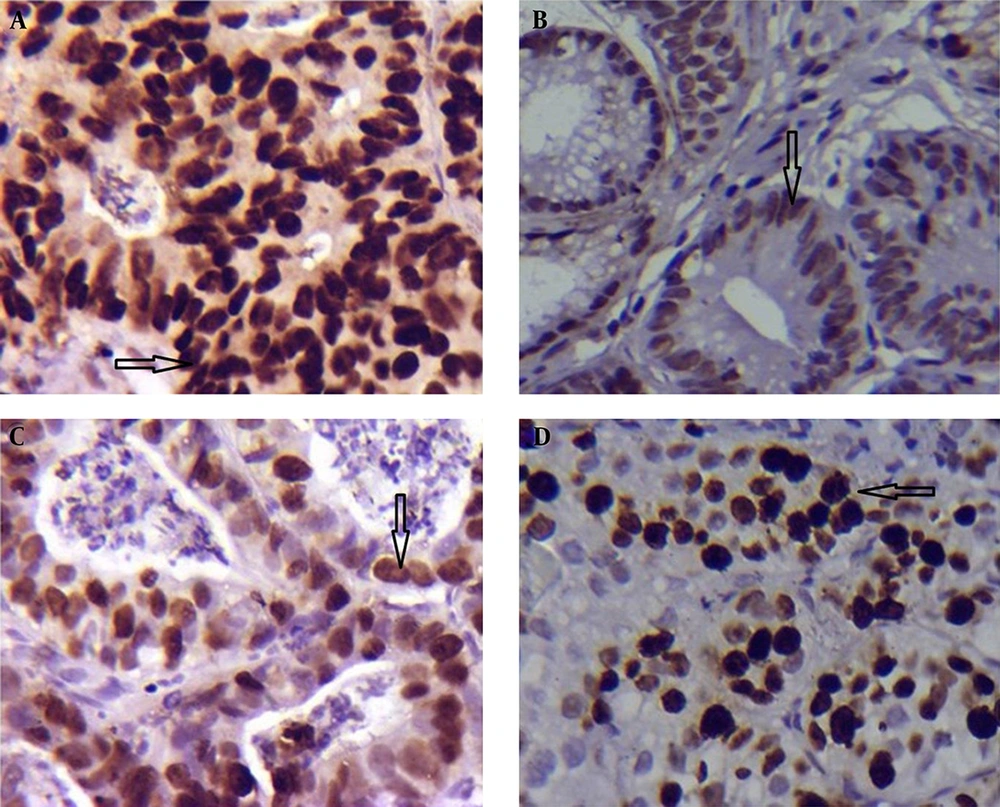
In GC, there was a significant different between the tissues infected and not infected with H. pylori regarding the expression of p53 (7.89 ± 0.85 vs. 4.13 ± 0.69; P = 0.001 < 0.05 Table 3; Figure 3A and B). There was no significant difference in Ki-67 expression between the groups with and without H. pylori infection (6.73 ± 0.60 vs. 4.95 ± 0.73; P = 0.097 > 0.05; Table 3; Figure 3C and D).
In the IM, DYS and GC there was a trend of progressive increase in positivity of p53 expression in the group with H. pylori infection; while Ki-67 expression in DYS specimens with H. pylori infection was lower than that of the IM group. In the group without H. pylori infection, from IM toward DYS the expression of the p53 and Ki-67 increased but in GC group the intensity of expression of both antibodies decreased (Table 3).
5. Discussion
The current study investigated the p53 and Ki-67 expression in the gastric epithelial lesions based on the infection with H. pylori. The study results showed that expression of p53 in the group with H. pylori infection was higher than that of the group without H. pylori infection (IM, DYS and GC). In addition, it was shown that from the IM to the GC, the p53 expression progressively increased in the group with H. pylori infection. In the current study, H. pylori were detected in 47.61% of IM, 50% of DYS and 45.23% of GC specimens. The lower prevalence of H. pylori in GC samples compared to that of the precancerous lesions justified as the modified gastric mucosa may become inhospitable for the continued infection with H. pylori and its colonization (10, 33).
The study data showed that in the IM group, the p53 expression in the specimens infected with H. pylori was higher than that of the specimens not infected with H. pylori. Zhang et al. (34) also showed that in the metaplasia phase, p53 expression in the group with H. pylori infection was higher than those of the group without H. pylori infection and normal mucosa. Similarly, Yang et al. (35) found that in IM, the expression of p53 in the specimens with H. pylori infection was higher than that of the specimens without H. pylori infection. In another study by Forones et al. (30), p53 was negative in all of the IM specimens, , and they suggested that mutation of p53 probably occurs in the latter stages of gastric carcinogenesis.
In the study, in DYS specimens the expression of p53 in those with H. pylori infection was higher than that of specimens without H. pylori infection. The findings were similar to the results reported by Zhang et al. (34) that p53 expression was higher in the specimens with H. pylori infection.
The current study showed that, in the GC group, the p53 expression in the tissues infected with H. pylori was higher than that of the tissues not infected with H. pylori. In line with the current study, Salih et al. (36) reported an overexpression of p53 in tumors infected with H. pylori. Li et al. (37) also reported that the GC tissue levels of p53 in the specimens infected with H. pylori were higher than those not infected with H. pylori. In another study, Zhang et al. (34) reported no expression of p53 in normal gastric mucosa, but there was an expression of p53 in GC tissues. Overexpression of p53 was observed in the specimens infected with H. pylori compared to those not infected with H. pylori. Accordingly, they concluded that H. pylori infection can boost p53 mutation. Morales-Fuentes et al. (38) reported that in the group with H. pylori infection, 91% of cases were positive for p53 expression while in the group without H. pylori infection, only 14% were p53 positive. Kubicka et al. (39) found more p53 mutations in patients with positive serology for H. pylori compared to the ones with negative test (43% vs. 14%). Yang et al. (35) showed no significant differences in p53 expression between patients with and without H. pylori infection in DYS and GC. Berloco et al. (40) also reported that p53 mutations were not related to H. pylori infection in patients with GC in an Italian population. On the other hand, Kodama et al. (41) showed that immunoreactivity for p53 in H. pylori infected group before eradication was significantly higher than that of non-infected group. After eradication of infection, the labeling index for p53 significantly reduced in both groups. This finding suggests that, the expression of p53 can be associated with H. pylori infection, and it can be concluded that H. pylori can be a risk factor for gastric carcinogenesis.
In the current study, the expression of Ki-67 in IM in the specimens with H. pylori infection was significantly higher than that of the specimens not infected with. Similarly, Jang et al. and Cabral et al. (42, 43) found that the expression of Ki-67 in IM was significantly higher in the group infected with H. pylori. This shows an agreement on the effect of H. pylori on the expression of Ki-67 during the precancerous phases. Some studies reported no statistically significant difference between Ki-67 expression in in the patients with and without H. pylori infection and IM (17, 44).
In DYS, expression of Ki-67 in the group with H. pylori infection was lower than that of the group without H. pylori infection. In GC, expression of Ki-67 was slightly higher in the specimens infected with H. pylori compared to those not infected with, but this difference was statistically insignificant.
Gucin et al. (14) reported that the level of Ki-67 expression in GC specimens had a positive relationship with H. pylori infection. Petersson et al. (45), in specimens with H. pylori infection, detected more than twofold and fourfold overexpression of Ki-67 and p53 respectively, compared to the uninfected subjects. Sasaki et al. (46) reported that in GC specimens Ki-67 labeling index in the group with H. pylori infection was higher than that of the group not infected with H. pylori. Van De Bovenkamp et al. (47) found that in the antral area, the number of proliferating cells and Ki-67 expression were significantly higher in the cases with H. pylori infection compared to those not infected with H. pylori. De Freitas et al. (48) investigated the effect of H. pylori infection on cell proliferation index and apoptotic index in gastric mucosa of the antrum and corpus parts. Their results showed that the expression of Ki-67 in the antral area significantly increased in the group with H. pylori infection, while apoptotic index increased in the gastric corpus area. There was no significant difference in apoptotic index between the groups with and without H. pylori infection. In the current study, biopsies were performed on different locations of the stomach; most of them were from antral part. This, as a limiting point, may affect the results. In patients with GC, there was no difference regarding Ki-67 expression between the groups with and without H. pylori infection. In this phase, Ki-67 overexpression is observed as a result of malignancy occurrence and this phase can be independent from H. pylori infection.
Since in IM phase the expression of Ki-67 was higher in the cases infected with H. pylori, it can be concluded that the presence of bacteria is essential for the onset of the disease (36). As observed in DYS, the expression of Ki-67 in the group with H. pylori infection was lower than that of the group without infection, and despite the fact that in GC there were no significant differences in the Ki-67 expression between the cases infected and not infected with H. pylori, it can be suggested that when the disease progresses from IM toward DYS and GC, the expression of Ki-67 is affected by genetic changes and molecular events that take place at the earlier stages of carcinogenesis and these changes can be caused by the colonization of H. pylori (38). The most important limitation of the study was insufficient biopsy specimens in group dysplasia, lack of some paraffin blocks and atrophy of some samples. Future studies are required to take larger sample sizes and samples from the lesions in the same locations of the stomach to clarify the differences observed between the current study and those of the previous researches. The current study showed a strong association between H. pylori infection and intensity of p53 expression. It suggests that H. pylori can be a risk factor to develop gastric cancer. Also, an association between H. pylori infection and overexpression of Ki-67 was observed in earlier stages of carcinogenesis. Further studies should be conducted on this matter.