1. Background
Salinity is one of the most significant stress factors affecting the growth and metabolism of plants and algae. Salt stress causes ionic imbalance in cells, leading to ionic toxicity and osmotic stress, which results in growth retardation directly by salt or indirectly through oxidative stress caused by reactive oxygen species (ROS). Salinity can cause the accumulation of large amounts of compatible solutes that produce enzymes and stabilize the structure of macromolecules and organelles (1). Salinity stress may alter the metabolic pathways of stressed organisms to enhance or induce biological activity. Algae are inherently divided into halophilic (needing salt for optimal growth) and halotolerant (having mechanisms to respond to and survive in highly saline environments) based on their salinity tolerance. In other words, halotolerant algae produce certain metabolites to protect themselves from salt stress damage (2). Since microalgae require resistance and adaptation to stressful conditions to survive, they may produce unique compounds due to metabolic changes (3). Therefore, changes in temperature, acidity, salinity, organic and inorganic compounds in the culture medium, as well as the availability of nutrients, effectively influence the synthesis of bioactive substances and antibacterial properties in microalgae (4). Plants have different biochemical mechanisms to cope with salinity stress, one of the most important being the production of osmotically active metabolites to control water flow. Under these stress conditions, the amount of carotenoids increases. For example, a study found that salt stress increased the biosynthesis and accumulation of carotenoids in Dunaliella spp. algae (5). Similarly, the levels of carotenoids, including lutein and beta-carotene, increased in Botryococcus braunii algae under salt stress and with rising salt concentrations (6). Additionally, the synthesis and accumulation of triacylglycerol in algae cells, along with increased changes in the composition of fatty acids and lipids, occur under stress conditions induced by chemical stimuli such as salinity, pH, and nutrient deficiencies (carbon and nitrogen sources) (7). Studies have shown that sodium chloride concentrations of 100, 200, and 300 mM significantly decrease the growth of Scenedesmus obliquus algae, but the amount of astaxanthin pigment increases as a response to this stress (8). In the study by Montazeri-Najafabadi et al. (9), increasing salinity led to a decrease in the growth of Dunaliella salina algae, and at higher salt concentrations, the production of beta-carotene as an intracellular secondary metabolite increased. Cyanobacteria are known for producing a variety of biochemically active natural products. Most cyanobacteria, such as Spirulina, Anabena, Nostoc, and Oscillatoria, produce various types of secondary metabolites and bioactive compounds (10). Spirulina platensis, a freshwater algae, is multicellular and filamentous, growing rapidly to a length of 0.5 to 1 mm. Spirulina can thrive in temperatures above 20°C and in waters with high salinity and alkalinity (pH 8.3 - 11), with the presence of carbonate, bicarbonate, and inorganic nitrogen (11). Although Spirulina can tolerate different levels of salinity, high salt stress can inhibit its growth and the electron transport activities of PSI and PSII (12-15).
Spirulina platensis is gaining attention not only for its nutritional value but also for its potential as a source of non-toxic drugs with therapeutic properties against anemia, tumor growth, and malnutrition (16). It has been reported that S. platensis and its extracts exhibit biological properties such as cancer prevention, cholesterol reduction, immune system stimulation, reduction of drug and toxic metal toxicity, and protection against radiation damage (17). These properties are attributed to various compounds including phenolics, phycobiliproteins, carotenoids, organic acids, sulfated polysaccharides, and unsaturated fatty acids. One study reported higher antioxidant activity in Spirulina extract compared to commercial Chlorella algae, due to its higher content of phenolic compounds (18).
Spirulina species have also demonstrated antibacterial (19) and antiviral activities (20). Researchers have reported that S. platensis extract inhibits the growth of pathogenic bacteria such as E. coli, Staphylococcus aureus, Salmonella Typhi, Pseudomonas aeruginosa, and Klebsiella pneumoniae (21). Additionally, salinity stress in S. platensis has been shown to increase or induce the production of biologically active compounds (22).
2. Objectives
The purpose of this study is to investigate the antibacterial activity of the methanolic extract of S. platensis algae cultivated under different salinity stresses against Y. rukeri, E. coli, Salmonella sp., and V. cholerae.
3. Methods
3.1. Preparation of Primary Stock and Cultivation of Spirulina Algae
The initial stock of S. platensis algae was obtained from Iran Algi Company (Mashhad). To cultivate the algae, 50 mL of algae stock in the linear phase of growth was added to 450 mL of water with salinity levels of 0, 3.5, and 7 ppt. Additionally, 16.8 g/L of edible sodium bicarbonate was added along with Zarouk culture medium. The cultivation was carried out under controlled and standard environmental conditions, including a temperature of 27 - 29°C, light intensity of 2000 lux, and aeration using an air pump (China, Hailea ACO-550) to prevent algae deposition (4.5 L/min).
3.2. Preparation of Dry Algae Powder
At the end of the cultivation period, the total algal biomass was collected using a spirulina algae harvesting net. The fresh biomass was then placed on a plate and dried in an oven (Binder, USA) at 45°C for 24 hours.
3.3. Preparation of Algae Extract
To prepare the algae extract, 2 g of spirulina algae powder was added to 100 mL of methanol solvent (Merck, Germany) (23). The mixture was then placed on a shaker at a speed of 100 - 130 rpm. After shaking, the extract was filtered through filter paper No. 42, and the solvent was separated using a rotary evaporator at 37°C. The residue was dried in an oven at 40°C and subsequently dissolved in dimethyl sulfoxide.
3.4. Preparation of the Studied Bacteria
The microorganisms used in this research included Yersinia ruckeri (KC291153), Vibrio cholerae (1611), Escherichia coli, and Salmonella sp. These were obtained from the General Directorate of Veterinary Medicine of Sistan and Baluchistan. The bacteria were cultured in nutrient broth medium and incubated for 24 hours at 30°C for Y. ruckeri, and at 37°C for V. cholerae, E. coli, and Salmonella sp. bacteria.
3.5. Minimum Inhibitory Concentration
The broth microdilution method was used to determine the minimum inhibitory concentration (MIC) of the extracts. First, 100 µL of sterilized Mueller Hinton broth (MHB) medium was distributed in all the wells of a 96-well microtiter plate. Then, 100 µL of algae extract at a concentration of 100 mg/mL was added to the first well, and serial dilutions were performed across the row. A negative control (MHB medium), a positive control (microbial suspension), and a culture control without the sample were also included. Next, 100 µL of bacterial inoculum, containing approximately 1.5 × 108 CFU/mL (0.5 McFarland standard), was added to all wells except the control wells. The plate was then incubated for 24 hours at 30°C and 37°C. The MIC was visually determined as the lowest concentration at which bacterial growth was negligible.
3.6. Minimum Bactericidal Concentration (MBC)
After determining the MIC of the extracts, 10 μL from all wells showing no visible bacterial growth was removed and cultured on nutrient agar (NA) media. The plates were then incubated for 24 hours at 37°C and 30°C. The MBC is defined as the lowest concentration of the antimicrobial agent that kills > 99.9% of the initial bacterial population, where no visible growth of bacteria is observed on the NA medium (24).
3.7. Agar Well Diffusion Method
The antibacterial activity of the methanolic extract of S. platensis on bacteria was investigated using the standard well diffusion method. For this purpose, Mueller Hinton agar medium was inoculated with microbial suspensions equivalent to the 0.5 McFarland standard using a sterile cotton swab. Wells with a diameter of 6 mm were then created using a sterile Pasteur pipette. Subsequently, 50 μL of methanol extract of spirulina algae at concentrations of 50, 25, 12.5, 6.25, 3.125, and 1.625 mg/mL were added to each well (25). The plates were incubated for 24 hours at 37°C and 30°C. After the incubation period, the diameter of the inhibition zones around the wells was measured with a millimeter ruler.
3.8. Statistical Analysis
SPSS software (version 16.0) and one-way analysis of variance (ANOVA) were used for data analysis. Duncan's test was employed to compare the means of different treatments. P-values less than or equal to 0.05 were considered statistically significant. Data were presented as mean ± standard deviation.
4. Results
4.1. Results of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Spirulina Algae Extract on Bacteria
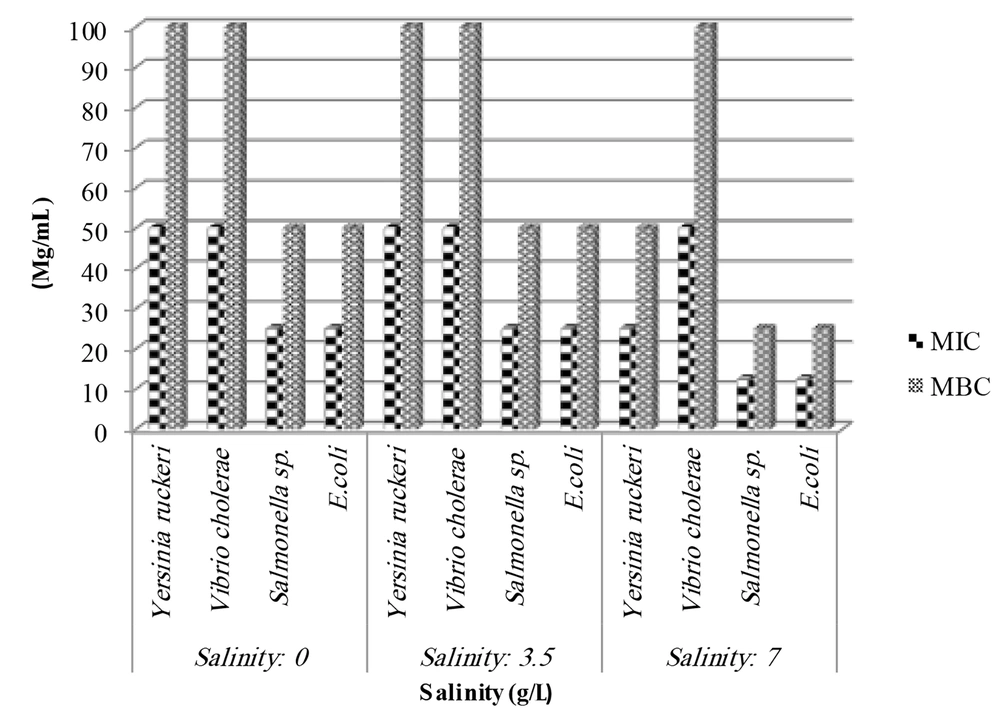
Table 1 and Figure 1 display the antibacterial activity of the algal sample against different organisms, including Y. ruckeri, E. coli, V. cholerae, and Salmonella sp. The results of the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration of the methanolic extract of Spirulina algae cultivated in three different salinities are summarized in Figure 1.
| Salinity (g/L) and Spirulina Platensis Extract (mg/mL) | Yersinia Ruckeri | Vibrio Cholerae | Salmonella | E. coli |
|---|---|---|---|---|
| 0 | ||||
| 50 | - | - | - | - |
| 25 | - | - | - | - |
| 12.5 | - | - | - | - |
| 6.25 | - | - | - | - |
| 3.12 | - | - | - | - |
| 1.56 | - | - | - | - |
| 3.5 | ||||
| 50 | 8.50 ± 0.50 a, BC | 8.50 ± 0.50 DE | 9.17 ± 0.29 a, BC | - |
| 25 | 7.83 ± 0.76 a, CD | 8.00 ± 1.00 ab,EF | 8.50 ± 0.50 b, C | - |
| 12.5 | 7.83 ±0.86 a, CD | 7.17 ± 0.29 bc,FG | 7.17 ±0.29 c, DE | - |
| 6.25 | 7.50 ± 0. 50 ab, CDE | 6.50 ± 0.50 c, G | 6.50 ± 0.50 d, EF | - |
| 3.12 | 7.50 ± 0. 50 ab, CDE | 0.00 ± 0. 00 d,H | 6.33 ± 0.29 d, F | - |
| 1.56 | 6.67 ± 0.29 b, E | 0.00 ± 0.00 d,H | 6.17 ± 0.29 d, F | - |
| 7 | ||||
| 50 | 9.83 ± 0.76 a, A | 11.00 ± 0.50 a,A | 10.83 ± 0.76 a, A | 10.50 ± 0.50 a |
| 25 | 9.33 ± 0.28 ab, AB | 10.17 ± 0.29 b,B | 9.83 ± 0.29 b, B | 9.17 ± 0.76 b |
| 12.5 | 8.50 ± 0.50 b, BC | 9.50 ± 0.50 bc,BC | 8.50 ± 0.50 c, C | 8.67 ± 1.04 bc |
| 6.25 | 8.50 ± 0. 50 b, BC | 9.16 ± 0.29 c,CD | 7.50 ± 0.50 d, D | 7.67 ± 0.76 cd |
| 3.12 | 8.50 ± 0. 50 b, BC | 7.83 ± 0.29 d, EF | 7.17 ± 0.29 de,DE | 7.17 ± 0.29 de |
| 1.56 | 7.33 ± 0.57 c, DE | 7.33 ± 0.58 d, FG | 6.50 ± 0.50 e, EF | 6.17 ± 0.29 e |
a The different superscripts in the same column indicate the significant differences (P < 0.05). Different uppercase letters indicate a significant difference between all concentrations of algal extract in two different salinities, and different lowercase letters indicate a significant difference between different concentrations of one salinity in each column.
As shown in the figure, the MIC and MBC of algae extract grown in salinity of 0 and 3.5 ppt against Y. ruckeri and V. cholerae were 50 and 100 mg/mL, respectively. For Salmonella and E. coli, the MIC and MBC were 25 and 50 mg/mL, respectively. However, algae extract grown in 7 ppt salinity stress exhibited lower MIC and MBC values, with 25 and 50 mg/mL for Y. ruckeri, and 12.5 and 25 mg/mL for Salmonella and E. coli.
Notably, the algal extract of different salinities did not show any variation in MIC and MBC values against V. cholerae. The extract grown in 7 ppt salinity demonstrated the maximum inhibitory effect against all tested species, while the methanol extract from salinities of 0 and 3.5 ppt showed little effect against the test organisms.
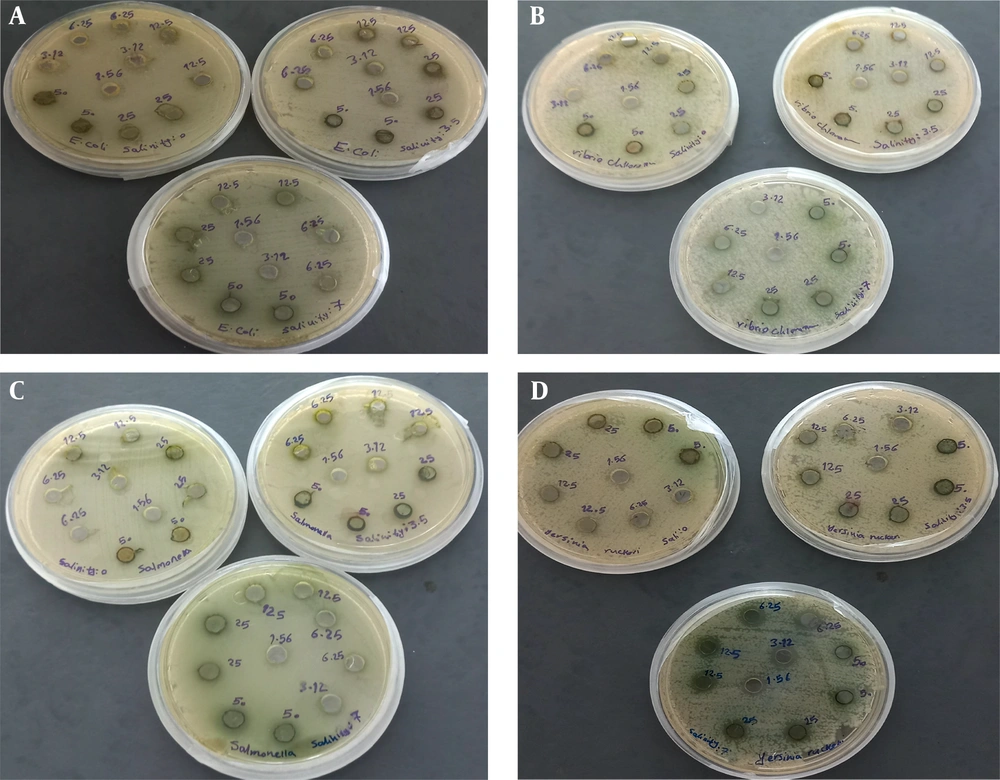
4.2. Results of Well Diffusion Method
The results related to the growth inhibitory zone of the studied bacteria by the methanolic extract of S. platensis algae grown in different salinities are presented in Table 1 and Figure 2. The data indicated that the concentrations of 50 and 25 mg/mL extract showed the largest inhibition zone diameter against Salmonella sp. bacteria (9.17 ± 0.29 mm and 8.50 ± 0.50 mm, respectively) compared to other bacteria. The lowest inhibition zone diameter was observed against E. coli bacteria.
In the treatment of algal extract grown at 7 ppt salinity stress, all studied concentrations of algal extract showed a higher inhibition zone diameter against V. cholerae compared to other bacteria. A significant difference was observed between the highest concentration and other concentrations across all bacterial strains in all treatments.
The comparison of different concentrations of algae extract between the two salinity stresses of 3.5 and 7 ppt for each bacterium showed a significant difference between the various concentrations of the extract in the two salinity stresses (P < 0.05). With increasing salinity, the diameter of the inhibition zone increased in all bacteria.
5. Discussion
Secondary metabolites of microalgae are molecules that do not play a role in the primary function of the cell but are involved in abiotic stress response, protection against enemies, signaling to other organisms, and communication with the environment. These metabolites also participate in various biological activities, including antibacterial activity (26 - 30). Consequently, stress can play an essential role in the production of antibacterial substances in microalgae. Previous studies have shown that Dunaliella salina algae collected from sewage-contaminated water exhibited more antibiotic activity compared to algae collected from less polluted areas (26). In the study by Cakmak et al. (27), Dunaliella algae exposed to high salinity increased beta-carotene production by 14%, suggesting the potential to produce metabolites with antibacterial activity. The results of the present study showed that with the increase in salinity stress, the antibacterial properties of methanol extracts of Spirulina algae against the studied bacteria also increased. Although the increase in salinity caused a decrease in the growth of S. platensis algae, it enhanced the antibacterial effects. This is consistent with the findings of Shalaby et al. (22), who investigated the effect of salt stress on the antiviral and antioxidant properties of S. platensis algae. Their results indicated that a salt concentration of 0.02 M (1.17 g of NaCL) slightly affected the growth of algae, while higher salinity levels (0.04 and 0.08 M or 2.34 and 4.68 g) led to a clear and progressive inhibition of growth, with a decrease in the dry weight of algae. The decreased growth in algae when exposed to salinity or other stresses is likely due to changes in algal metabolism to produce substances involved in salt tolerance and defense mechanisms. With increasing salt concentration, the production of phycocyanin and phycoerythrin increased, and salinity stress in S. platensis resulted in the increase or induction of biologically active compounds.
The findings of Najafi Qaghelestani and Najafi (28) also support the results of the present study. Their study showed that increasing the salinity stress level from 1.5 to 8 decimeters/second in Ocimum basilicum L. plants decreased the yield of fresh leaf weight but increased the efficiency of essential oil production. Salinity stress caused changes in the concentration of constituents of O. basilicum L. essential oil and effectively increased the control of gram-negative bacteria (E. coli, Pseudomonas aeruginosa, and Salmonella enterica) and gram-positive bacteria (Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus) compared to the control treatment (0.3) in both well diffusion and microdilution methods. This aligns with the results of the present study.
Additionally, the results of this study are consistent with those of Kilic et al. (29), who reported that increasing the salinity percentage from 10% to 20% in the culture medium of D. salina algae enhanced the antibacterial activity of the chloroform extract of the algae in the disk diffusion method. In the study by Mehdipour et al. (30), the antimicrobial activity of the hydroalcoholic extract of the microalgae Scenedesmus sp. and S. platensis grown in the BG-11 culture medium and the wastewater from the desalination facility of the Bandar Turkmen unit in Golestan province was investigated. The goal was to inhibit the growth and proliferation of S. aureus and E. coli. The results, obtained within 24 hours, showed that in the extract of Scenedesmus sp. and S. platensis grown in BG-11 medium and 50% and 100% effluent dilutions, there was no minimum inhibitory concentration after 24 hours against S. aureus and E. coli bacteria. Spirulina grown in 100% wastewater, compared to Scenedesmus, showed a constant trend and exhibited more controlling properties against S. aureus in the final hours.
The difference between Mehdipour's results and our results could be attributed to the type of extract used. Many studies on S. platensis algae have indicated that methanolic extract, which was used in the present study, has high antimicrobial properties (21, 31). Additionally, the type of culture medium used for cultivating Spirulina algae influences its antibacterial properties. In the present study, the Zarrouk culture medium was used, while the mentioned study used the BG-11 medium. Antibacterial activity depends on many factors, including the species of algae, solvents used, microorganisms, season, and conditions of growth and maintenance of algae (32-34). Furthermore, the quality of secondary metabolites depends on the type of algae, environmental conditions, and artificial culture medium (35).
Studies have shown a direct relationship between the concentration of the extract and the diameter of the inhibition zone in all cases. This means that by reducing the concentration of the extract, the diameter of the inhibition zone decreased (36, 37). The results of our study also revealed that with the increase in the concentration of the extract from 1.625 to 50 mg/mL, the diameter of the inhibition zone of bacteria also increased. In the salinity treatments of 3.5 and 7 ppt, the highest concentration of the extract showed the largest diameter of the inhibition zone against all bacteria.
5.1. Conclusions
Our results showed that increasing the level of salinity stress from 3 to 7 ppt increased the antibacterial efficiency of the methanolic extract of cultivated S. platensis compared to the control treatment (0 ppt salinity). Further studies with higher salinities are suggested to determine the optimal concentration of salinity for this purpose. Due to the lack of freshwater resources, growing Spirulina algae in salt water is a cost-effective method. Additionally, applying salt stress can be an effective way to produce more secondary metabolites in Spirulina algae for use in the pharmaceutical industry.