1. Background
One of the big problems that new medicine has brought with it, despite its apparent advantages over traditional medicine, is the increasing use of chemical drugs, which unfortunately is becoming more acute day by day. In the meantime, the increasing spread of antibiotic resistance to Staphylococcus aureus species is one. These are the problems that doctors deal with. Staphylococcus aureus has been known as one of the important human pathogens for many years. Infections caused by this bacterium occur permanently and frequently in hospitalized patients (1), and despite antibiotic treatment, they leave severe complications.
Staphylococcus aureus is one of the most common bacteria causing hospital infection in infectious diseases such as endocarditis, osteomyelitis, and food poisoning (2). With the presence of beta-lactam-resistant S.aureus, a new antibiotic named methicillin was prepared from penicillin. Methicillin-resistant strains of Staphylococcus aureus (MRSA) are considered a serious threat in hospital infections, which make the treatment process of this bacterial infection difficult and cause many clinical epidemiology problems in hospitals. This organism is transmitted between hospitalized patients and staff (3).
The frequency of MRSA strains in Asian countries such as China, Korea, and Taiwan is more than 70%, and in Iran, it is about 50%. Methicillin-resistant strains of Staphylococcus aureus is responsible for 29% of nosocomial infections and nearly 50% of deaths in New York hospitals (4). Rhazya stricta plant belongs to the Apoyanaceae family (5) and Rauwolfioideae subfamily (6). Ashurak is known as Asfand in Ascending Arabia. Ashurak is a small evergreen shrub. This plant is used as medicine in the treatment of various diseases in the countries of Afghanistan, India, Iran, Iraq, Pakistan, Qatar, and Saudi Arabia. Natural dyes have been known for thousands of years and have been used to make and dye fibers. Carpet weavers used this dye to dye carpet threads, but the production and expansion of synthetic dyes limited the use of natural dyes (7).
The use of natural dyes has problems such as the complexity of the dyeing process, the problems of reproducing a color, limited shades, and inappropriate characteristics of color stability. To solve the mentioned problems, different natural dyes, different grains, and other effective parameters of rose color can be used (8).
2. Objectives
The aim was to study investigating the antimicrobial activity and pigmentation of the extract of R.stricta plant against human bacteria.
3. Methods
3.1. Cultivation and Identification of Staphylococcus aureus and Methicillin-Resistant Strains of Staphylococcus aureus
Cultured samples included blood samples. Eight samples of Staphylococcus aureus were isolated from patients. The pure strains obtained on the culture medium were identified using catalase tests and gram staining at the genus level. Finally, they were identified by performing the coagulase test by tube and slide method and checking the formation of agglutination, as well as positive mannitol fermentation of S.aureus species (9).
To isolate MRSA, a growth test was used on mannitol salt agar containing 6 μg/mL oxacillin with salt (4% mass/volume). After cultivation and incubation for 24 hours at 35°C, eight strains were identified as methicillin-resistant Staphylococcus aureus and MRSA strains.
3.2. Preparation of the Extract
Maceration (soaking) method was used to prepare the extract. For this purpose, after crushing the leaves of the plant, 50 grams of the sample was soaked and kept in methanol-ethanol-ethyl acetate-water percent for 48 hours. Then the obtained extract was concentrated with a smooth filter paper and using a vacuum distillation device (rotary).
3.3. Determining the Dry Weight of the Extract
First, the weight of a test tube was determined and one milliliter of the extracted extract was transferred into it. Then the tube containing the extract was dried at room temperature. The difference in the weight of the tube was equivalent to one milliliter of extract. The average of three repetitions was calculated as the dry weight of the extract, and then it was dissolved in DMSO solvent and stored at 4°C until use.
3.4. Measuring the Minimum Inhibitory Concentration of the Extract Against Methicillin-Resistant Strains of Staphylococcus aureus and Staphylococcus aureus
To measure minimum inhibitory concentration (MIC), the microbroth dilution method based on the CLSI protocol with some changes was used (10). In order to measure the antimicrobial activities of Razia extract against bacteria, the initial concentration of 200 mg/mL extract was prepared in DMSO solution (Made in Germany, Merck factory). A 96-well plate (made in USA, Sigma factory) and mueller hinton broth culture medium were used for the experiment.
Bacterial suspension was prepared with the help of McFarland half standard. The volume of 100 microliters of bacterial suspension was added to all the wells except the negative control and the first concentration. Then the cultured microplates were kept at 35°C and examined after 24 hours of incubation. The positive control well contained liquid culture medium of bacterial suspension and the maximum concentration of solvent, while the negative control well contained only culture medium and solvent. After the incubation time, the tested microplates were studied. The formation of sediment at the bottom of the well was considered as a sign of bacterial growth and its absence as a sign of bacterial no-growth.
The concentration of the first no-growth well was the minimum no-growth concentration or MIC. To obtain MBC, 10 μL of the MIC concentration and previous concentrations were spot cultured on mueller hinton agar. All cultured plates were incubated at 35°C for 18 hours. After this period of time, the desired plates were examined, and the concentration of the first spot culture that showed no growth was considered as MBC.
3.5. Rose Color Parameters
In this research, the effect of dyeing parameters such as dyeing percentage, type of tooth, type of acid, and dyeing method on the shade obtained from the dyeing of Ashurak in woolen yarn has been investigated. For experimental tests, woolen yarn obtained from merino fibers with a grade of 5 meters and 100 twists per meter was used.
In this research, the effect of dyeing parameters such as dyeing percentage, type of tooth, type of acid, and dyeing method on the shade obtained from the dyeing of Ashurak in woolen yarn has been investigated. For experimental tests, woolen yarn obtained from merino fibers with a score of 5 meters and 100 warps per meter was used. For the rose color, the woolen product was first washed in a 2% solution of non-ionic textile soap at 40°C for 20 minutes. Then, they were dyed using simultaneous over-dentation and post-dentation methods in baths with different materials and percentages of Ashurak, acid, and dentine. According to the investigation of polygenetic properties, different teeth including potassium aluminum double sulfate, iron sulfate, copper sulfate, potassium dichromate, zinc chloride, nickel chloride, and tin chloride were used in an amount of 5% relative to the weight of the product. Also, to determine the effect of the type of acid, rose color was performed in the presence of different acids such as acetic, sulfuric, tartaric, oxalic, and citric acid. In order to determine the effect of temperature and concentration on absorption, absorption spectrophotometry and Beer-Lambert's law were used.
4. Results
The results of this study showed that the lowest inhibitory concentration of ethyl acetate extract against S.aureus was 25 ppm, and 3 strains were inhibited at this concentration. The lowest inhibitory concentrations of aqueous, ethanolic, methanolic, and hydroalcoholic extracts were 12.5, 12.5, 12.5, and 3.1 ppm, respectively (Table 1).
| Strain Bacteria | Ethyl Acetate MIC/MBC | Water MIC/MBC | Ethanol MIC/MBC | Methanolic MIC/MBC | Hydroalcoholic MIC/MBC |
|---|---|---|---|---|---|
| S1 | 50/100 | 12.5/25 | 12.5/25 | 12.5/25 | 12.5/25 |
| S2 | 50/100 | 25/50 | 12.5/25 | 25/50 | 25/50 |
| S3 | 25/50 | 25/50 | 25/50 | 50/100 | 12.5/25 |
| S4 | 50/100 | 12.5/25 | 25/50 | 12.5/25 | 25/50 |
| S5 | 50/100 | 25/50 | 25/50 | 50/100 | 3.1/6.25 |
| S6 | 50/100 | 12.5/25 | 25/50 | 50/100 | 3.1/6.25 |
| S7 | 25/50 | 25/50 | 12.5/25 | 25/50 | 25/50 |
| S8 | 25/50 | 12.5/25 | 25/50 | 25/50 | 50/100 |
Abbreviations: MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration.
The lowest inhibitory concentration of ethyl acetate against MRSA samples was equal to 25 ppm. Meanwhile, the lowest inhibitory concentrations of aqueous, ethanolic, methanolic, and hydroalcoholic extracts were 12.5, 12.5, 12.5, and 25 ppm, respectively (Table 2).
| Strain Bacteria | Ethyl Acetate MIC/MBC | Water MIC/MBC | Ethanol MIC/MBC | Methanolic MIC/MBC | Hydroalcoholic MIC/MBC |
|---|---|---|---|---|---|
| MRSA1 | 25/50 | 12.5/25 | 12.5/25 | 12.5/25 | 50/100 |
| MRSA2 | 25/50 | 25/50 | 50/100 | 25/50 | 25/50 |
| MRSA3 | 50/100 | 12.5/25 | 25/50 | 50/100 | 50/100 |
Abbreviations: MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration.
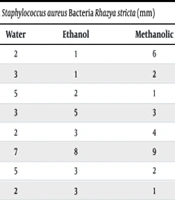
The results of the diameter of the inhibition zone showed that the maximum diameter of the inhibition zone of Ethyl Acetate (2 mm), aqueous (7 mm), ethanolic (8 mm), methanolic (9 mm), and hydroalcoholic (10 mm) extracts against S. aureus (Table 3).
| Strain Bacteria | Ethyl Acetate | Water | Ethanol | Methanolic | Hydroalcoholic |
|---|---|---|---|---|---|
| S1 | 1 | 2 | 1 | 6 | 2 |
| S2 | 2 | 3 | 1 | 2 | 6 |
| S3 | 2 | 5 | 2 | 1 | 2 |
| S4 | 2 | 3 | 5 | 3 | 5 |
| S5 | 1 | 2 | 3 | 4 | 1 |
| S6 | 1 | 7 | 8 | 9 | 10 |
| S7 | 1 | 5 | 3 | 2 | 5 |
| S8 | 1 | 2 | 3 | 1 | 2 |
The results of this study showed that the smallest diameter of the inhibition zone was related to the ethyl acetate extract of Razia against MRSA (2 mm), while the largest diameter of the inhibition zone was related to the methanolic extract of Razia (8 mm) (Table 4).
| Strain Bacteria | Ethyl Acetate | Water | Ethanol | Methanolic | Hydroalcoholic |
|---|---|---|---|---|---|
| Mrsa1 | 2 | 5 | 4 | 6 | 7 |
| Mrsa2 | 0 | 7 | 2 | 8 | 3 |
| mrsa | 1 | 3 | 2 | 4 | 5 |
5. Discussion
Antibiotics are valuable drugs for the treatment of many human diseases; however, excessive use of these drugs will lead to microbial resistance. Therefore, scientists have prioritized research on different parts of medicinal plants to discover new drugs of plant origin (11).
In the study of Hassan et al., the growth inhibitory properties of the ethanolic and methanolic extracts of the medicinal plant R.stricta against K.pneumoniae strains were evaluated using the MIC method and disk diffusion. In addition, the biofilm inhibition potential of these extracts was investigated using crystal violet. HPLC analysis identified 19 components divided into 6 flavonoids, 11 phenolic acids, stilbene (resveratrol), and quinol, and showed variations in the number of components and their amounts between the extracts. Both extracts showed interesting antibacterial properties against K.pneumoniae isolates. The two extracts also showed strong biofilm inhibitory activities, with inhibition percentages ranging from 81.5% to 98.7% and from 35.1% to 85.8% for the ethanolic and methanolic extracts, respectively. Rhazya stricta leaf extract showed strong antibacterial and antibiofilm activity against K.pneumoniae isolates and can be a good candidate for the treatment or prevention of K.pneumoniae-related infections (12).
In another study, which was conducted with the aim of synthesis, identification, and antibacterial activity of silver nanoparticles using the medicinal plant Ashurak, the results showed that the average size of the synthesized silver nanoparticles was 20 nm with a spherical shape. Nanoparticles based on R.stricta showed improved antibacterial activity against gram-positive and gram-negative strains (13).
In the study of AL-Sahli et al., who investigated the fungicidal properties of nanoparticles synthesized in the medicinal plant Ashurak, the results showed that the mycelium growth of all species was inhibited along with severe ultrastructural changes. Silver nanoparticles were the most effective fungicides (14). In the study of Fazeli-Nasab et al., they investigated the effects of different solvents for the extraction of phytochemicals from R.stricta on the activity of Salmonella Typhimurium. The lowest MIC against Salmonella Typhimurium was obtained from hydroalcoholic solvent with 1.3 ppm. The most effective extraction solvent to inhibit the growth of S. Typhimurium was the hydroalcoholic type with an average growth inhibition zone diameter of 12.25 mm, followed by ethanol extract with a growth inhibition zone diameter of 12.12 mm (15).
In the study by Khan et al., who investigated the antibacterial activity of R.stricta leaf extract against multidrug-resistant human pathogens, antimicrobial activities of different concentrations of five solvent extracts (aqueous alkaloid, aqueous non-alkaloid, organic alkaloid, organic non-alkaloid, and complete aqueous extracts) derived from R.stricta leaves against several pathogenic and multidrug-resistant bacteria, including MRSA and extended-spectrum beta-lactamase-positive Escherichia coli, were analyzed. In laboratory conditions, molecular and electron microscopy analyses conclusively showed the antimicrobial effects of these extracts on a panel of gram-negative and gram-positive bacteria. Organic alkaloid extract was the most effective extract against E.coli and MRSA bacteria, leading to cell membrane disruption as observed by transmission electron microscopy (16).
5.1. Conclusions
The results of the study showed that the medicinal plant with different solvents is a good inhibitor of pathogenic bacteria and can be used in the treatment of infection.