1. Background
The olive tree has long been valued for the beneficial properties of its fruit and leaves (1). What distinguishes the olive tree (Olea europaea L.) from other plants are its valuable properties and unique compounds. The polyphenolic compounds in olive leaves play a prominent role in controlling blood pressure, strengthening the immune system, improving heart health, and increasing the body's energy level (2, 3).
The Mediterranean region is renowned for producing extra virgin olive oil. The olive tree has grayish-green, spindle-shaped leaves (generally about 5 - 6 cm long and about 1 - 1.5 cm wide in the middle of the leaf) with fine margins and short petioles. The proportions of these may vary depending on the age of the tree, the growing conditions, and local pruning practices (2).
Olive leaf extract (OLE) is a dark brown liquid with a bitter taste that is recognized in natural medicine for its wide range of health benefits. This extract is traditionally used as a herbal supplement because it contains polyphenolic compounds with beneficial properties, such as increasing energy levels. In addition to the benefits mentioned above, OLE also has antimicrobial properties. The composition of the extract may vary according to different conditions, such as geographical location, cultivar, and plant nutrition (4).
Olive leaf oil also contains other compounds, such as triterpenes, flavonoids, and chalcones, which offer therapeutic benefits, including antimicrobial properties (5). Studies show that olive leaf polyphenols have a greater variety and quantity than the phenols found in olive oil, thus giving olive leaf oil more health benefits (6, 7). The structure of olive phenols bears a remarkable similarity to estrogen receptors, and it is believed that this similarity affects the control of cancers caused by hormonal imbalances (8, 9). Olive leaf extract may be effective in regulating inflammatory responses and oxidative stress pathways by acting as a phytoestrogen (10, 11).
Interleukin-1, interleukin-6, and TNF-α play roles in immune health pathways and interact with many molecules. These cytokines can have both pro-inflammatory and anti-inflammatory roles (12). Research on the effect of olive extract on different types of cells, animal models, and human models shows that the compounds in OLE can influence the inflammatory fate of cells by altering the levels of different cytokines (13, 14). A diet containing olive leaf oil can affect the expression of a network of proteins involved in the acute phase response, lipid transfer, cholesterol homeostasis, and blood coagulation. Retinol-binding protein 4 is one of the proteins whose expression is altered by olive leaf oil.
The mouse preadipocyte cell line 3T3-L1 is a commonly used tool to analyze the intracellular pathways involved in preadipocyte differentiation (a process commonly known as adipogenesis). This cell line is typically used to study cellular and molecular mechanisms related to insulin resistance and diabetes in laboratory settings.
Modern lifestyles contribute to increased oxidative stress through factors such as poor diet, pollution, smoking, and lack of physical activity. This makes finding natural, accessible ways to combat oxidative stress more crucial than ever. Olive leaf extract has a long history of traditional use as a natural remedy, suggesting potential therapeutic properties that warrant scientific investigation. The rich polyphenol content of OLE makes it a promising source of natural antioxidants.
Belief in the healing properties of OLE stems primarily from historical beliefs, and while there is some scientific documentation, its mechanism of action and the certainty of its benefits require further research.
2. Objectives
This study was conducted to investigate the antioxidant effect of OLE in adipose tissue fibroblast cell line (3T3-L1) and its impact on inflammatory pathways and RBP4 inflammatory gene expression.
3. Methods
Olive leaves were collected from Garmsar city in the spring of 2013. After washing with distilled water to remove impurities, they were spread in the shade to dry and then powdered. The soaking method was used to extract the olive leaves, and the hydroalcoholic extract was obtained using a ratio of 30% distilled water to 70% ethanol. The supernatant was stored at -80°C until use (15).
3.1. Chemicals
The adipose tissue fibroblast cell line (3T3-L1) was obtained from the Pasteur Institute in Tehran, Iran. Fetal bovine serum (FBS), Dulbecco's Modified Eagle Medium (DMEM), and antibiotics (penicillin and streptomycin) were purchased from Gibco. IL-1, IL-6, TNF-α cytokine ELISA kits and the MDA assay kit were purchased from Karmania Parsgen, Iran. The precision of the ELISA method was pg/mL². The primers were prepared by Pishgam Iran.
3.2. Cell Culture
In the first step, 100,000 cells/mL of adipose tissue fibroblast cells (3T3-L1) were cultured under sterile conditions in DMEM medium containing 10% FBS at 37°C and 5% CO2. Then, the cells were exposed to OLE concentrations of 5, 10, 500, and 4000 μg/mL. The cells were evaluated daily for morphology (degree of adhesion to the substrate), possible contamination, and color change of the cell culture medium.
3.3. MTT Assay
3 x 104 3T3-L1 fibroblast cells were seeded in each well of a 96-well plate, and the culture medium was adjusted to a final volume of 100 microliters. After 24 hours of incubation at 37°C, 5% carbon dioxide, and 80% humidity, the old culture medium was removed, and different concentrations of OLE (1 to 2000 μg/mL) were added. Following incubation for 24 and 48 hours, 5 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) solution at a concentration of 5 mg/mL was added to each well. After the formation of dark blue crystals, the contents of each well were aspirated, and after washing with PBS, 100 μL of DMSO was added.
To completely dissolve the precipitate, the plate was incubated for 2 - 4 hours in the dark at room temperature. The culture medium alone was used as a blank, and the culture medium containing cells was used as a control. The optical absorbance of the wells was measured at 570 nm using a plate reader (Biotek, USA). This experiment was repeated three times, with three wells (triplicates) used for each concentration.
3.4. Assessment of Antioxidant Capacity by DPPH Method
The DPPH method was used to assess the antioxidant activity of the OLE. Briefly, 20 microliters of OLE were diluted to a volume of 1 mL with absolute ethanol and added to 1 mL of DPPH solution. The optical density of the solution was measured at a wavelength of 517 nm, and the antioxidant activity was calculated according to the kit instructions by comparing the absorbance to a standard curve. Vitamin C, known for its high antioxidant activity, was used as a positive control in this experiment.
3.5. Measurement of Malondialdehyde
Malondialdehyde (MDA) measurement is commonly used as an indicator of lipid peroxidation. In this study, the MDA assay kit from Carmania Parsgene was used according to the manufacturer's instructions. The protocol involves the reaction of thiobarbituric acid with MDA, resulting in a pink color. The absorbance of the solution was measured using a spectrophotometer (Analytik Jena, Jena, Germany) at a wavelength of 534 nm.
3.6. Measurement of Reactive Oxygen Species and Catalase Levels
To measure free oxygen species (ROS), DCFDA or DCHF and a flow cytometry device were used. Catalase activity was measured using a spectrophotometric method through the removal of peroxides. To measure the level of catalase, hydrogen peroxide was added according to the instructions provided in the Parsgen Carmania kit.
3.7. Measurement of IL-1, IL-6, and TNF-α Levels
The amount of inflammatory cytokines IL-1, IL-6, and TNF-α in the supernatant solution of treated cells was measured using a Parsgen Carmania ELISA kit (Iran), with an accuracy of 2 pg/mL.
3.8. Ribonucleic Acid Extraction and Polymerase Chain Reaction (RT-PCR)
Ribonucleic acid extraction was performed using TRIzol reagent (Invitrogen, USA) from both control and treated groups. The quantity and quality of the extracted RNA were checked using a Nanodrop device (Thermo SCIENTIFIC) and electrophoresis, respectively. 500 ng of RNA was converted into cDNA using a reverse transcription kit (Amplisense, Russia, К3-4-100-CE). RT-PCR reactions were performed using an Applied Biosystems 7500 machine (USA, ABI) with SYBR Green (RealQ Plus 2x Master Mix Green Amplicon) under the following conditions: Denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 30 s. The internal gene actin beta (ACTB) was used to normalize the results. The sequences of the primers used are shown in Table 1.
| Genes | Sequence (5'->3') | Product Size | |
|---|---|---|---|
| ACTINB | F: 5‘→3‘ | ACCCAGCACAATGAAGATCAAGA | 175 |
| R: 5‘→3‘ | ACAGTCCGCCTAGAAGCATTTG | ||
| RBP4 | F: 5‘→3‘ | GCCTCTTTCTGCAGGACAAC | 110 |
| R: 5‘→3‘ | GCACACGTCCCAGTTATTCA | ||
3.9. Statistical Analysis
Data were expressed as mean ± standard deviation, and one-way ANOVA was used to compare data in GraphPad Prism version 8 software. To check for the existence of a linear relationship between the amount of olive leaf hydroalcoholic extract and the levels of cytokines under investigation, a linear regression test was used, and P < 0.05 was considered statistically significant.
4. Results
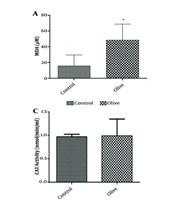
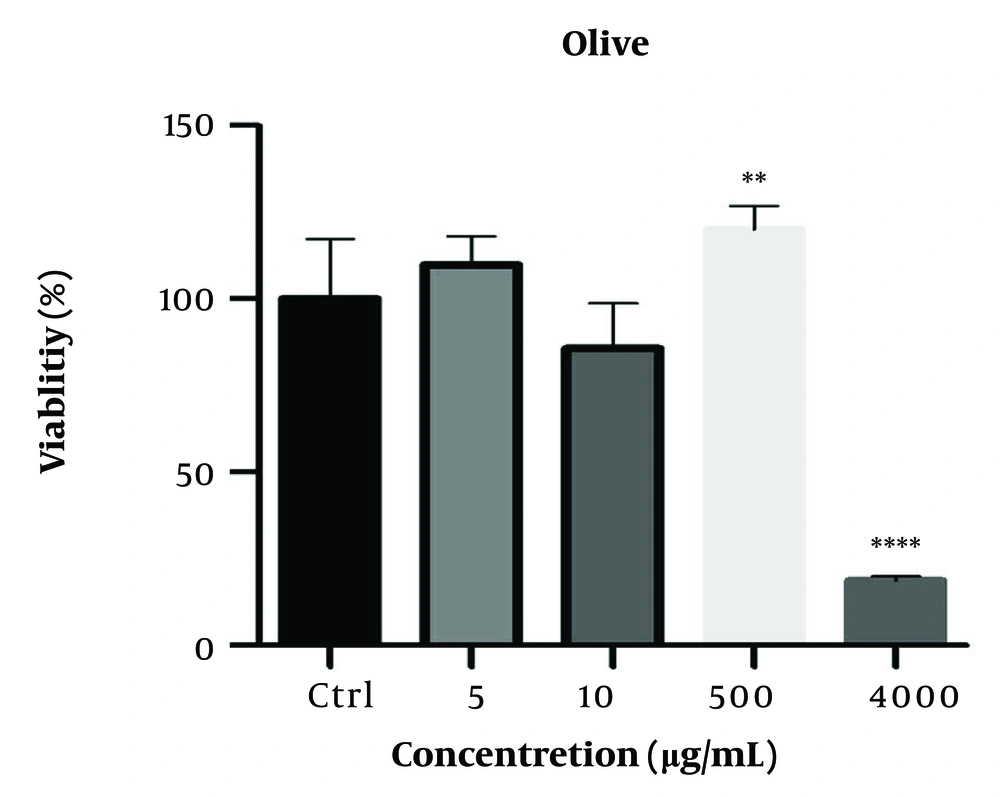
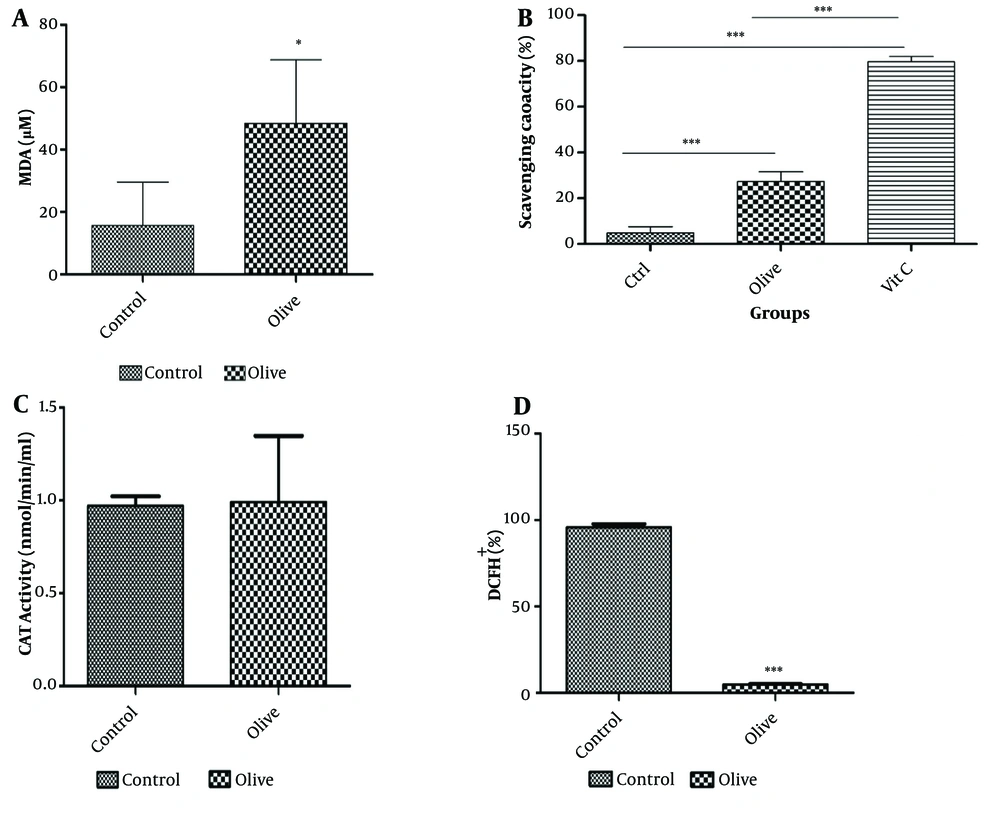
The viability of 3T3-L1 cells was evaluated after treatment with concentrations of 1, 100, 1000, and 2000 µg/mL OLE for 24 and 48 hours (Figure 1). The MTT assay shows that the hydroalcoholic extract of olive plant leaves has a concentration-dependent effect on cell proliferation (**P ≤ 0.05). The measurement of malondialdehyde (MDA) as an index of oxidative stress, the analysis of antioxidant capacity by the DPPH radical method, and the results of the tests for the activity of catalase and ROS are shown in Figure 2. The results showed that the increase in catalase enzyme activity was not additive (P < 0.05). As shown in Figure 2, the active oxygen level in the group treated with OLE decreased significantly (P ≤ 0.001***).
The effect of Olive leaf extract (OLE) on the viability of 3T3-L1 cells after 24 hours of incubation with cells evaluated by the MTT method. This graph shows that the hydroalcoholic extract of olive plant leaves has a concentration-dependent effect on cell proliferation. ∗∗P ≤ 0.05 indicates the significance of the results compared to the control. ∗∗∗∗ indicates that the result is significantly different from the control.
Evaluation of antioxidant and peroxidation effect of olive leaf extract on 3T3-L1 cell line. A, olive leaf hydroalcoholic extract significantly increased the amount of MDA produced by fibroblast cells (∗P ≤ 0:05); B, 2,2-diphenyl-1-picrylhydrazyl (DPPH) molecules can measure total cellular and mitochondrial reactive oxygen species levels. Comparing the antioxidant activity of Olive leaf extract (OLE) with the known antioxidant compound vitamin C shows the acceptable antioxidant capacity of OLE (∗∗∗P ≤ 0:001); C, catalase activity did not show significant changes in the presence of OLE (P < 0.05); D, reactive oxygen level significantly decreased in OLE-treated group (∗∗∗P ≤ 0:001).
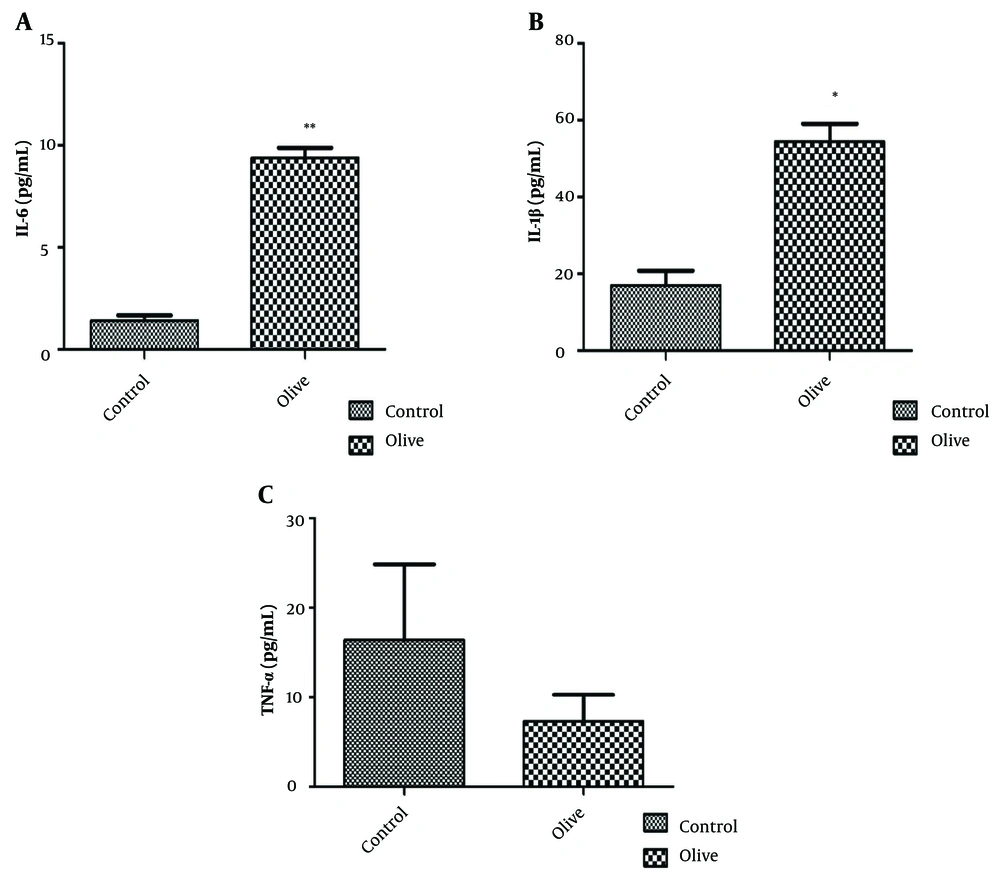
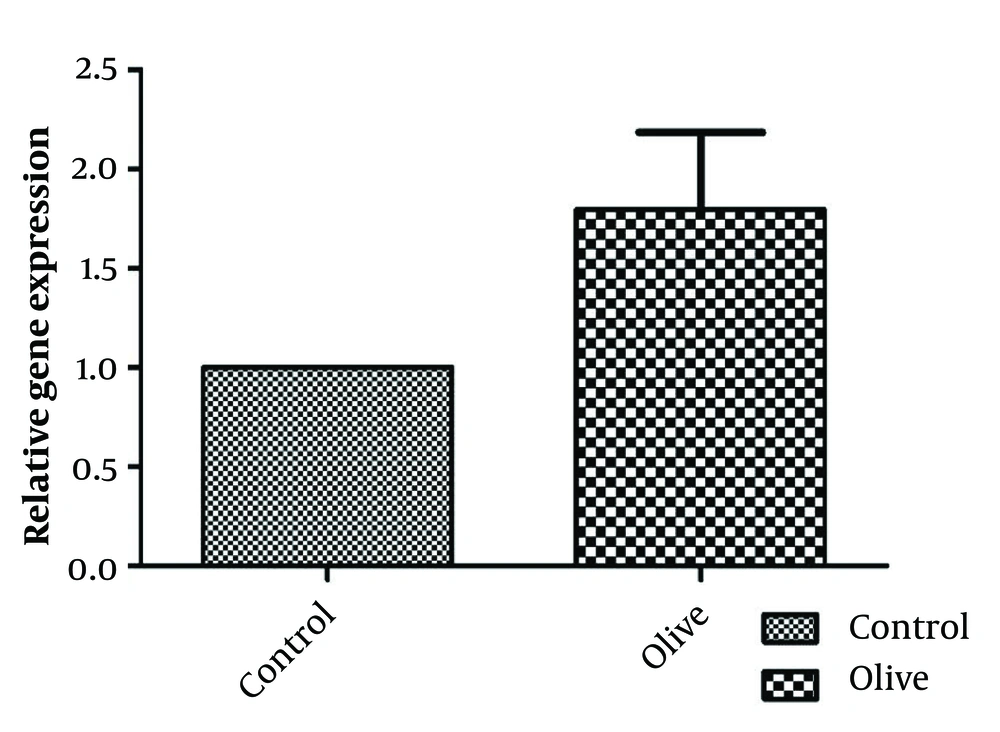
The results of investigating the inflammatory effect of OLE are shown in Figure 3. Analyses show a significant increase in the level of interleukin 1 and 6 and a decrease in the pro-inflammatory cytokine TNF-α in the fibroblast cell line after treatment with OLE. The levels of IL-1 and IL-6 in the group treated with olive extract show significant differences (P < 0.05 and P < 0.01, respectively) compared to the control group. The amount of TNF-α also decreased, but it was not significant (P > 0.05). Evaluation of the effect of OLE on the control and treatment groups shows that the relative expression of the Retinol Binding Protein 4 (RBP4) gene increased compared to the control group; however, this increase was not significant (Figure 4).
5. Discussion
Many studies have investigated the effect of olives and their extracts on oxidative pathways, inflammatory reactions, and the complications of various diseases. However, less research has explored the effect of Garmsar OLE on the genes of inflammatory pathways in 3T3-L1 cells. Given that this cell line is related to fibroblastic cells of fat tissue, it is often used in studies investigating antioxidant, antibiotic, and insulin resistance activities (16).
In this study, the impact of the hydroalcoholic extract derived from olive plant leaves on adipose tissue fibroblast cells was examined. Following exposure of the cell line to the extract's effective concentration, biochemical assays were conducted to evaluate antioxidant capacity and peroxidation-related parameters.
The results demonstrated that the peroxidation capacity of cells in the treated group was significantly increased. The antioxidant capacity test also revealed that the level of active oxygen was significantly decreased in the presence of OLE. While the catalase test result was not statistically significant, the combined data from the antioxidant and peroxidation tests suggest that OLE has a high capacity for regulating these pathways in fat tissue, indicating its potential for controlling related disorders in the Garmsar region.
Hadrich et al. have demonstrated that OLE contains various compounds, the most notable of which is oleuropein. Their research findings indicate that OLE can inhibit the accumulation of lipid droplets in 3T3-L1 cells. Additionally, they observed increased expression of the Glut-4, IRS1, p85-PI3K, and p-Akt genes and decreased levels of the cytokines TNF-α and IL-6 in 3T3-L1 cells (17). The reduced level of pro-inflammatory cytokine TNF-α in 3T3-L1 cells treated with OLE suggests anti-inflammatory properties. This is significant, as chronic inflammation in adipose tissue contributes to insulin resistance and other metabolic complications.
Possessing numerous antioxidant properties, OLE holds great potential as a food supplement. Lins et al. have demonstrated that OLE is rich in phenolic and flavonoid compounds, with oleuropein being the primary active ingredient responsible for its antioxidant properties. Studies have shown that OLE can protect human red blood cells from oxidative damage (18).
The oxidative stress pathways trigger the production of numerous inflammatory cytokines, including TNF-α, a key pro-inflammatory cytokine. Several studies have shown that OLE can reduce TNF-α expression. Our findings corroborate these previous observations, demonstrating that the TNF-α level was decreased at a concentration of 2000 μg/mL (Figure 3c). This suggests that, like any other effective substance, the use of OLE should be limited, as excessive consumption may be associated with adverse effects.
Obesity is considered a chronic inflammatory condition, and the immune system attempts to enhance its response by regulating the release of various cytokines to combat various disorders. Studies have demonstrated that OLE can both decrease and increase the expression of cytokines (19, 20). Our data showed that 3T3-L1 adipocytes treated with OLE exhibited a significant increase in the expression of IL-1 and IL-6 genes, suggesting the activation of the immune system in response to adipose tissue inflammation and the initiation of a defense mechanism (Figure 3a, b).
Additionally, given the normal characteristics of adipose tissue cells (3T3-L1), it can be inferred that excessive consumption of OLE may lead to immune system dysfunction and potentially trigger inflammation. Consequently, it is likely that olives, like any other substance, should be consumed in moderation.
This study provides the first measurement of the effects of Garmsar native olive on 3T3-L1 cells. To enhance the validity of these findings, further research is recommended, including investigations at the clinical level and studies involving other native olive varieties within the country.
5.1. Conclusions
Overall, the findings of this study indicate that OLE possesses potent antioxidant properties, making it a potential candidate for use in the food industry and as a natural and safe dietary supplement. However, our research also suggests that high doses of OLE may have paradoxical effects, potentially disrupting inflammatory pathways and inducing the expression of inflammatory genes in normal cells. Further studies are warranted to elucidate the dose-dependent mechanisms of action of olive extract and to determine its optimal dosage for various applications.