1. Background
Bacopa monnieri L. Penn., commonly known as 'Brahmi,' holds significant medicinal and economic value within the Scrophulariaceae family. Initial phytochemical analysis identified the presence of alkaloids, saponins, flavonoids, tannins, carbohydrates, proteins, and anthraquinones. Quantitative estimation revealed that various parts of B. monnieri contain alkaloids in the root, stem, and leaf; saponins in the root, stem, and leaf; and flavonoids in the stem and leaf. This finding provides valuable pharmacobotanical information critical for the accurate identification and authentication of the crude drug and its botanical components (1).
Bacopa monnieri is one of the 32 medicinal plants prioritized for research and development by the National Medicinal Plant Board (NMPB), Government of India. Due to its medicinal properties, Brahmi has a strong market demand, with an estimated annual consumption of 1000 tons in India. More than 90% of the plant material utilized by the industry is sourced from the wild (2).
Research has demonstrated that B. monnieri extract possesses various therapeutic properties, including anti-depressant, anti-anxiety, anti-inflammatory, antipyretic, analgesic, sedative, free radical scavenging, and anti-lipid peroxidative activities (3). It also exhibits anti-cancer properties effective against multiple cancer types, including prostate, breast, colon, liver, and neurological cancers. Bacosides from B. monnieri extract demonstrate anti-mutagenic, cytotoxic, anti-inflammatory, and anti-cancer activities. Additionally, B. monnieri extract has shown free radical scavenging activities in vitro on human lymphocytes without exhibiting genotoxic activity (4).
The most important active ingredients in B. monnieri include flavonoids, steroid compounds, alkaloids such as bromine and herpestine, and triterpene saponins known as bacosides (5). The saponins in B. monnieri are a combination of bacosides A and B. The plant also holds clinical significance for improving immune disorders in humans (6). As an antioxidant, B. monnieri enhances the activity of superoxide dismutase and glutathione peroxidase. Research further indicates that bacoside A exhibits potential anti-cancer effects by inducing cell cycle arrest and apoptosis in glioblastoma multiforme (5).
Initially, synthetic drugs and surgery were the primary methods for cancer treatment. However, due to its potent antioxidant capacity and therapeutic effects, B. monnieri has gained attention for its potential against a range of diseases, including cancer. Standardized extracts of the plant have demonstrated efficacy against clastogens and carcinogens. These bioactive compounds offer a promising, non-toxic, and cost-effective approach for treating cancer and improving health outcomes (4).
Natural resources of B. monnieri are limited, chemical synthesis is unfeasible, and seed propagation is restricted due to limited seed availability and viability. Plant tissue culture techniques provide an alternative method for the production of secondary metabolites in this plant (7). Traditional cultivation methods are time-consuming, as the plant requires several years to mature and achieve the desired level of metabolite production. Plant tissue cultures offer numerous advantages over conventional cultivation methods, including independence from geographical and seasonal variations, year-round production, relatively short growth cycles, mass propagation of uniform, high-quality plant material, and the absence of pesticides and herbicides (8).
Various approaches are employed to enhance the yield of secondary metabolites in plant cell culture, including the use of plant growth regulators (PGRs), elicitors, precursor addition, and optimization of the culture environment (9).
2. Objectives
The primary objective of this research is to investigate the effect of PGRs on the elicitation of flavonoid and polyphenol content in B. monnieri tissue culture and to examine their impact on the apoptosis of normal and cancerous skin cells.
3. Methods
3.1. Tissue Culture and Sample Preparation
The study was conducted by the Iranian Research Organization for Science and Technology (IROST). To enhance the production of secondary metabolites, MS medium was supplemented with five different types of PGRs: NAA, IBA, kinetin, BAP, and 2IP, each applied in concentrations ranging from 0 to 1 mg/L.
3.2. Extraction of Samples
The flavonoid extraction method followed the procedure described in (10). The samples were oven-dried at 50℃ and then ground using a mortar and pestle. A total of 0.25 g of finely dried powdered sample was extracted with 20 mL of 60% (v/v) aqueous ethanol (Merck, Germany). The extracts were agitated at 25°C for 48 hours.
3.3. Biochemical Analysis
3.3.1. Determination of Phenolic Content
The levels of phenolic compounds in the ethanolic extract of B. monnieri leaves were quantified using the Folin-Ciocalteu reagent assay (11). One mL of the extract in methanol was mixed with 5.0 mL of Folin-Ciocalteu reagent and 4.0 mL of sodium carbonate. The mixture was shaken thoroughly and incubated in darkness for 1 hour. The absorbance was then measured at 750 nm using a spectrophotometer. A standard curve was prepared using gallic acid monohydrate as the reference standard. The total phenolic content was calculated and expressed as mg of gallic acid equivalent per g of dry weight of the extract (mg GAE/g DW).
3.3.2. Determination of Flavonoids Content
Total flavonoids were measured following the methods described by (12) and (13). The extraction of flavonoids from B. monnieri required 80% ethanol, which facilitated the release and extraction of flavonoids. Quantitative determination was performed using the AlNO₃ reagent as outlined by (14). A standard curve was prepared using quercetin, and the absorbance of the samples was measured at 510 nm using a spectrophotometer. Three replicate samples were analyzed under identical conditions, and the total flavonoid content was calculated based on the standard curve.
3.4. Cell Lines
Cancer and normal cell lines used in this study were sourced from the Iranian National Center for Genetic and Biologic Resources. The cell lines included human fibroblast cells (HDF; IBRC No. C10506) and skin cancer cells (A375; IBRC No. C11337). The cells were cultured in DMEM culture medium supplemented with penicillin, streptomycin, and 10% FBS. The incubation conditions were maintained at 37°C, with 5% CO₂ and saturated humidity.
3.5. MTT Assay
The viability of both normal and cancerous skin cells was evaluated after exposure to varying concentrations (0.025, 0.5, 1, 2, 4 µg/L) of a treatment containing 0.4 mg/L 2IP, 0.8 mg/L kinetin, 25 mg/L chitosan, 1 mg/L NAA, 1 mg/L IBA, 0.8 mg/L BAP, and 25 mg/L phenylalanine for 48 hours. This assessment was performed using the MTT test, which evaluates mitochondrial function through the reduction of MTT.
The MTT viability test [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was conducted as described by (15), with modifications. The MTT assay is a quantitative colorimetric method used to measure cell viability. It involves the reduction of a yellow tetrazolium salt into dark blue formazan by mitochondrial dehydrogenases in viable cells. This reaction occurs only when mitochondrial function is intact.
After incubation, the cell medium was replaced with fresh medium (100 µL/well), followed by the addition of 50 µL of MTT solution (5 mg/mL in PBS) to each well. The plates were then incubated for 4 hours at 37°C. Formazan crystals formed during the reaction were dissolved by adding 100 µL of DMSO to each well, followed by mixing and incubation for an additional 4 hours. The concentration of formazan was determined by measuring optical density at 570 nm.
Cytotoxicity was calculated based on the survival rate of cells compared to the control group, with the survival rate of control cells set at 100%.
3.6. Statistical Analyses
The experiments were conducted in triplicate, and the results are presented as mean ± MSE. Quantitative data analysis was performed using statistical software (SPSS 16, IBM, USA). The findings revealed a significant accumulation of flavonoids and polyphenols in treated in vitro cultures compared to untreated samples after four weeks of culture. A one-way analysis of variance (ANOVA) was performed, followed by Duncan's multiple-range test to compare mean values. A significance level of P < 0.05 was considered statistically significant.
4. Results
4.1. Effects of Different Plant Growth Regulator on the Secondary Metabolite of Bacopa monnieri
Table 1 and Figure 1 illustrate the response of secondary metabolite production in B.monnieri to various PGRs, including 2,4-D, NAA, IBA, BAP, and kinetin, applied individually at concentrations ranging from 0.0 to 1 mg/L. The results indicate that these hormones positively influenced metabolite production in B. monnieri.
| Source of Variation | df | Mean of Square | |
|---|---|---|---|
| Flavonoids (mg/g/DW) | Phenolic(mg/g/DW) | ||
| BAP concentration | 5 | 0.03 a | 36.05 a |
| Error | 12 | 0.009 | 3.806 |
| Kinetin concentration | 5 | 0.013 a | 17.911 a |
| Error | 12 | 0.008 | 2.881 |
| 2IP concentration | 5 | 0.014 a | 23.371 b |
| Error | 12 | 0.007 | 5.306 |
| IBA concentration | 5 | 0.049 a | 44.121 a |
| Error | 12 | 0.008 | 4.605 |
| NAA concentration | 5 | 0.041 a | 44.703 a |
| Error | 12 | 0.007 | 4.106 |
Analysis of Variance for Plant Growth Regulator Effect on Secondary Metabolite
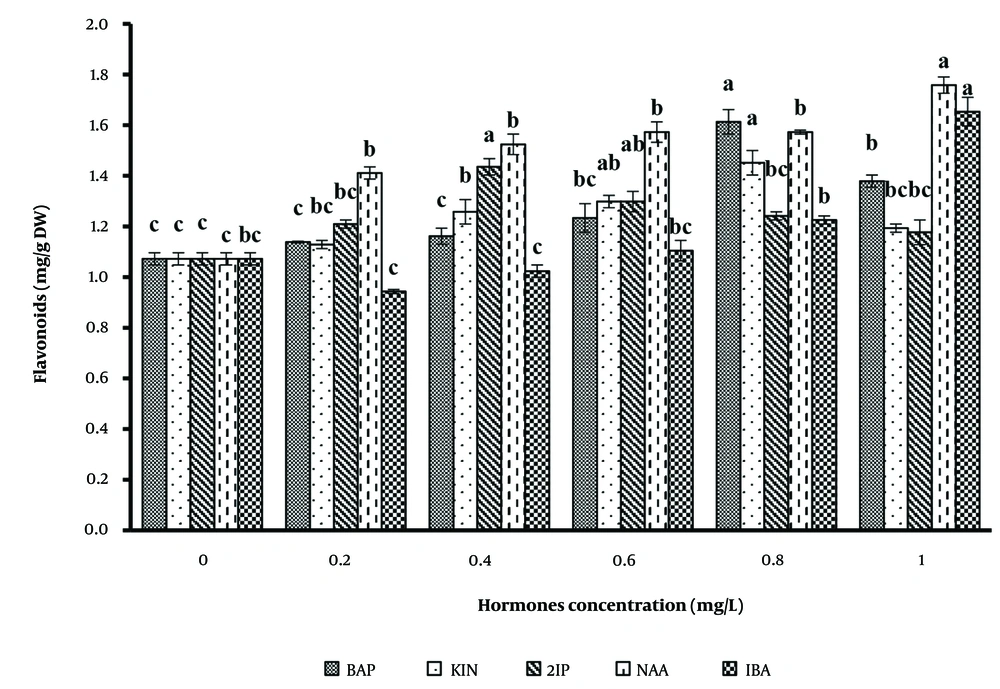
The pattern of flavonoid production was analyzed using different auxins and cytokinins on basal MS medium. The data revealed that the effects of hormones on flavonoid production were significantly greater than the control. Among the auxins tested (Figure 2), 1 mg/L NAA and IBA produced the highest flavonoid levels, at 1.76 and 1.65 mg/g DW, respectively. For cytokinins, the medium containing 0.8 mg/L BAP showed the most successful flavonoid production compared to other concentrations.
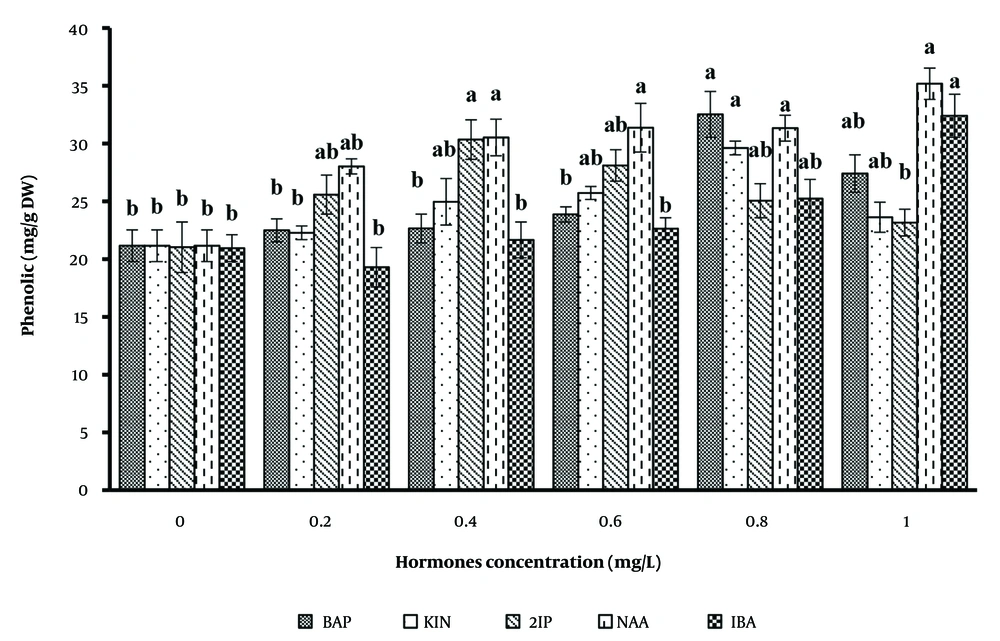
The effects of IBA and NAA at various concentrations on polyphenol accumulation revealed that the highest accumulation occurred with 1 mg/L NAA, followed by IBA. Even in the control medium, the polyphenol content was 21.26 mg/g DW, which was significantly lower than the 1 mg/L NAA medium. Additionally, the medium containing 0.8 mg/L BAP produced the highest polyphenol content among cytokinins tested.
Figure 3 highlights the variation in polyphenol productivity, which peaked at 35.17 ± 0.24 mg/g DW with 1 mg/L NAA, followed by IBA at 32.39 ± 0.31 mg/g DW. In contrast, cytokinins resulted in lower productivity. Interestingly, an increase in plant growth regulator concentration led to a decrease in polyphenol content, though higher biomass levels resulted in increased secondary metabolite productivity.
Previous studies support these findings. For example, in Ipomoea batatas suspension culture, exposure to 2,4-D concentrations ranging from 0 to 4 mg/L over two weeks caused a gradual reduction in anthocyanin concentration, from 29% to 14.1%, respectively (16). Similarly, in Rudbeckia hirta, the accumulation of Pulchellin E varied with different auxins (IAA, 2,4-D, and IBA), with 2,4-D demonstrating the strongest enhancement of secondary metabolites. Conversely, NAA inhibited Pulchellin E biosynthesis (17). A study on H. bonariensis reported that 2,4-D enhanced flavonoid production, while NAA produced the lowest amount. A similar comparison between NAA and IBA was observed in Artemisia absinthium.
4.2. Effect of Different Extracts from Bacopa monnieri on Human Fibroblast Cells and A375 Cell Lines
In this experiment, the effects of various treatments, including 2IP (0.8 mg/L), kinetin (0.8 mg/L), IBA (1 mg/L), NAA (0.8 and 1 mg/L), BAP (0.8 mg/L), chitosan (3 mg/L), and phenylalanine (20 mg/L), were investigated on A375 skin cancer cells compared to normal HDF fibroblast cells. Concentrations ranging from 0.25 to 4 μg/mL of each treatment were applied to the cells for 48 hours.
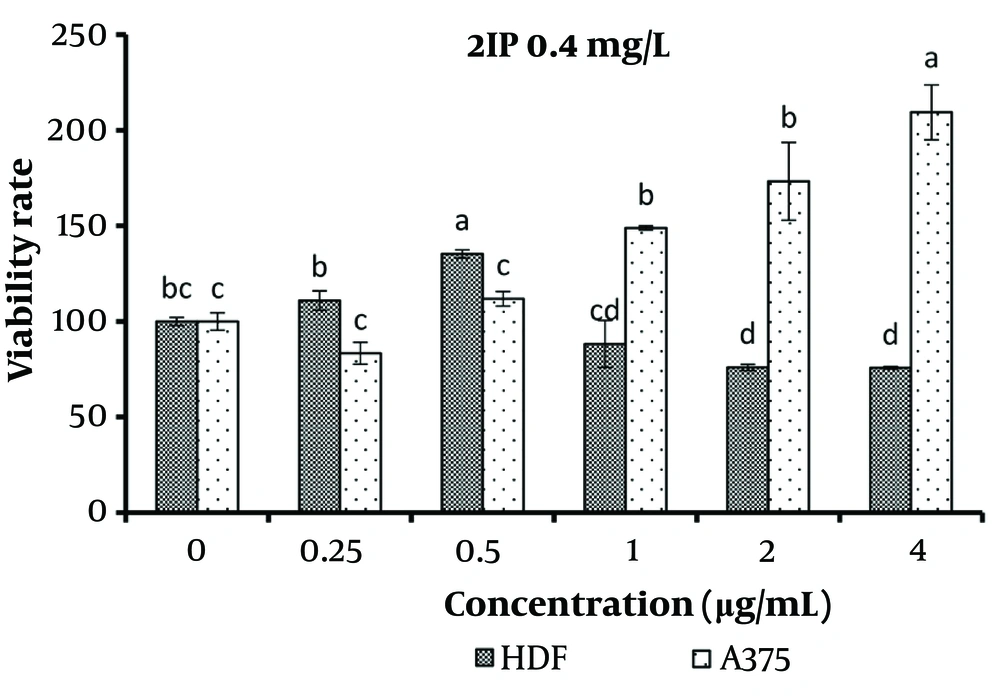
The results revealed that a concentration of 0.5 μg/mL of 2IP (0.4 mg/L) significantly increased the survival of normal cells compared to the control (P < 0.05). However, concentrations equal to or greater than 2 μg/mL significantly increased the survival of normal cells as well (P < 0.05). Additionally, concentrations exceeding 1 μg/mL significantly increased the survival of cancer cells compared to concentrations ranging from 0 to 0.5 μg/mL (P < 0.05). Therefore, only the 0.5 μg/mL concentration significantly enhanced the survival of normal cells without affecting the survival of cancer cells (P < 0.05) (Figure 4).
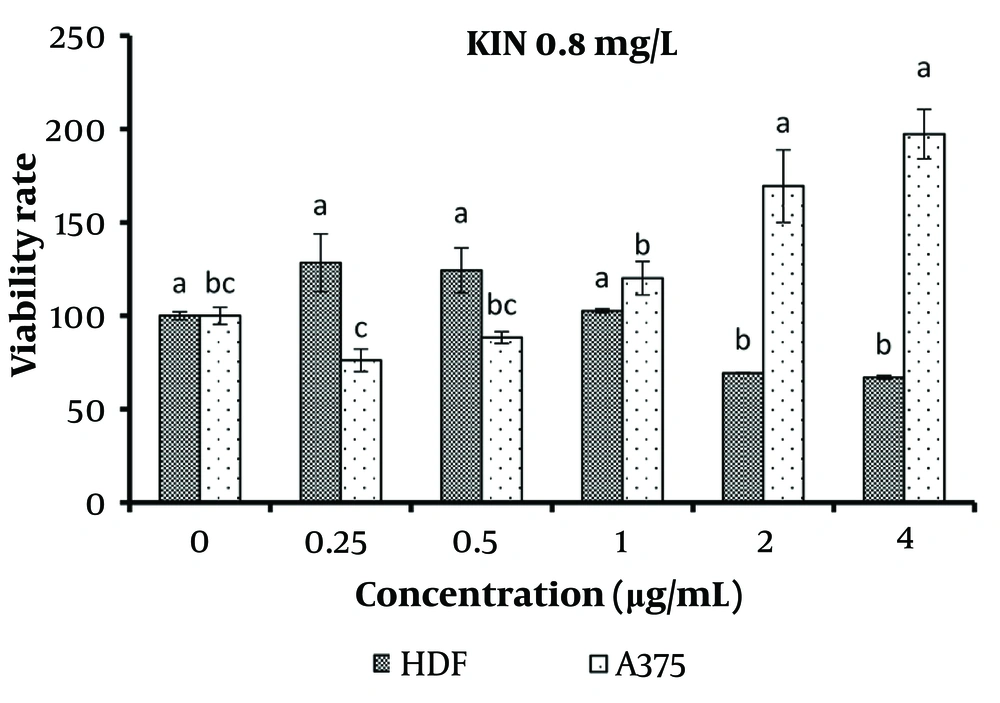
The results of kinetin (0.8 mg/L) treatment on normal and cancerous skin cells are presented in Figure 5. Concentrations ranging from 0.25 to 1 μg/mL of kinetin showed no significant difference in the viability of normal cells compared to the control. However, concentrations exceeding 2 μg/mL significantly reduced the viability of normal cells (P < 0.05). For cancer cells, concentrations from 0.25 to 1 μg/mL of kinetin treatment exhibited no significant difference in viability compared to the control, while concentrations equal to or greater than 2 μg/mL significantly increased cancer cell survival (P < 0.05).
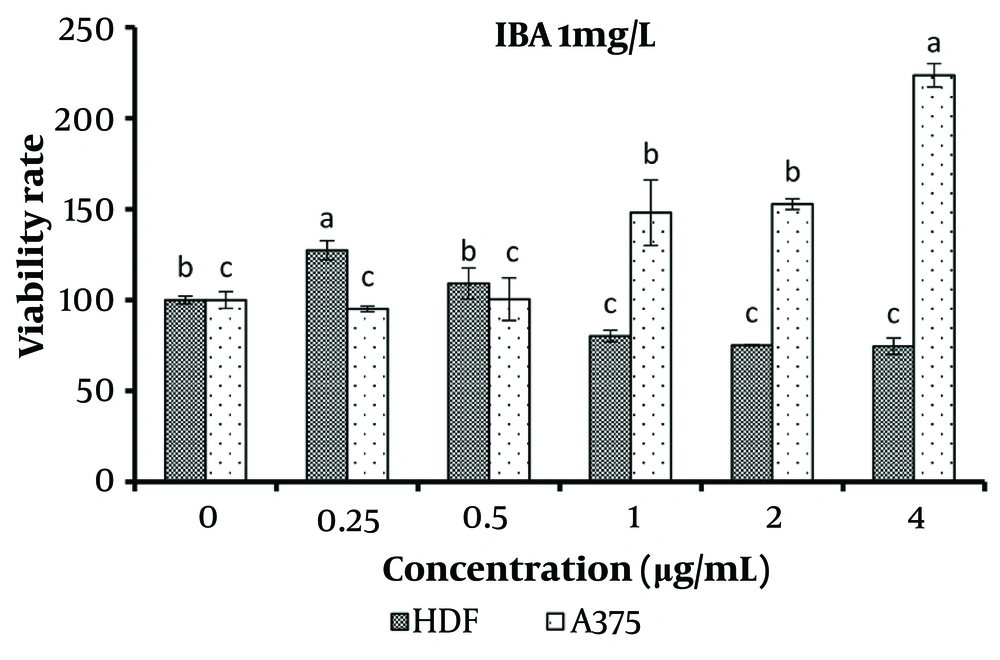
The concentration of 0.25 μg/mL of the treatment containing 1 mg/L IBA significantly increased the viability of normal cells (P < 0.05). However, concentrations equal to and greater than 1 μg/mL significantly decreased the viability of normal cells while simultaneously increasing the viability of cancer cells (P < 0.05) (Figure 6).
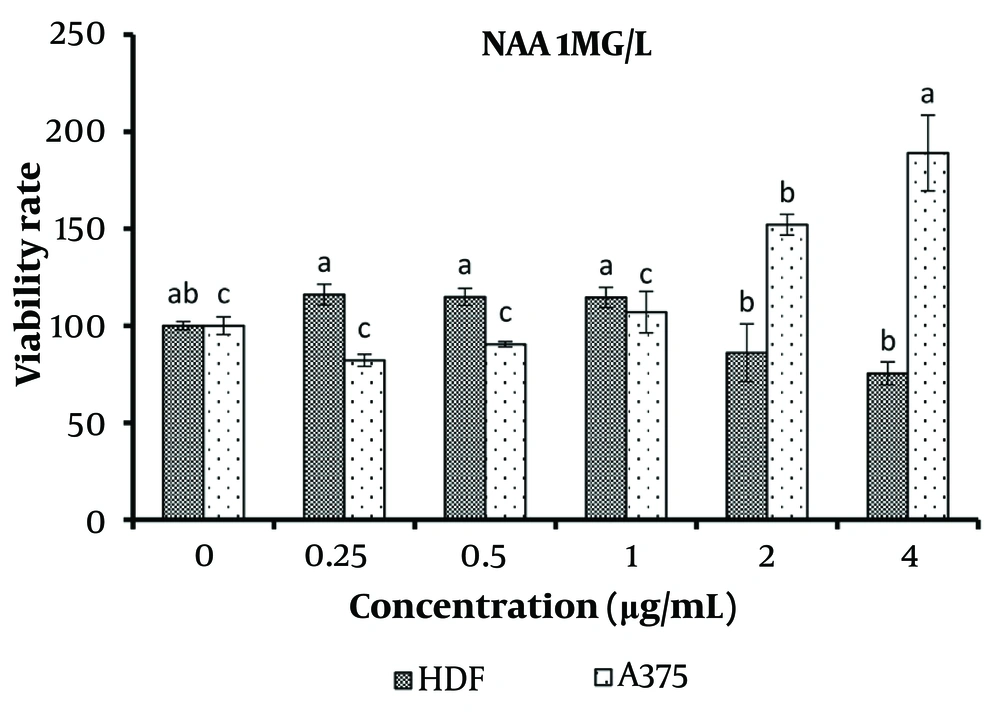
For the treatment with 1 mg/L NAA, concentrations ranging from 0.25 to 1 μg/mL did not show any significant difference in viability compared to the control for both normal and cancer cells. However, concentrations of 2 and 4 μg/mL caused a significant decrease in the viability of normal cells and a significant increase in the viability of cancer cells (P < 0.05) (Figure 7).
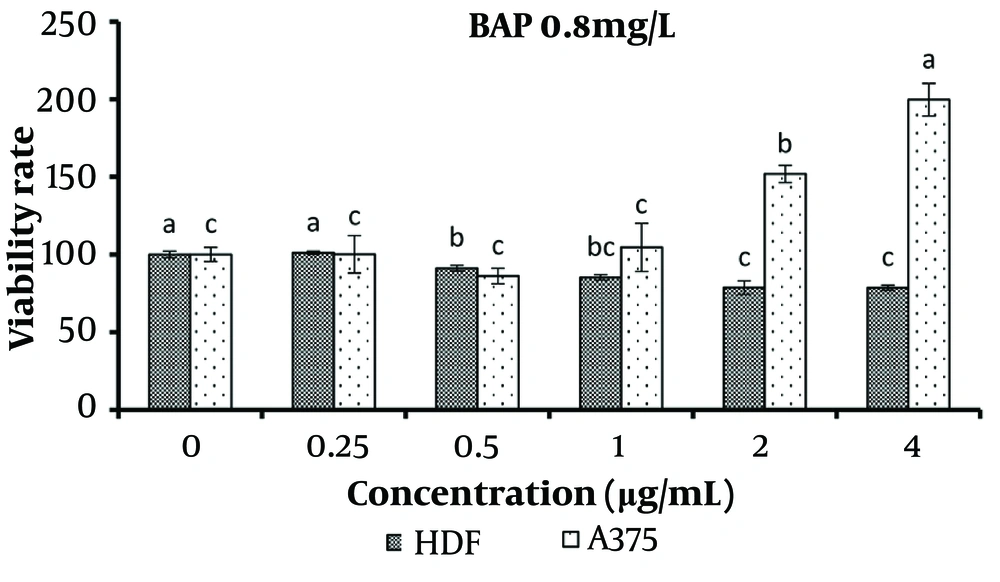
Increasing the treatment concentration of 0.8 mg/L BAP from 0.25 to 4 μg/mL resulted in a significant decrease in the viability of normal cells (P < 0.05). Conversely, concentrations of 2 and 4 μg/mL significantly increased the survival of cancer cells compared to other treatments (P < 0.05) (Figure 8).
5. Discussion
The results of using effective hormone concentrations in B. monnieri plant cultures demonstrated that when concentrations of 2 μg/mL or higher of the specified extracts were applied to the culture medium of normal and cancerous skin cells, there was a significant increase in the number of cancerous cells and a concurrent decrease in normal cells.
No tolerable upper Dietary Reference Intake (DRI) has been established for flavonoids, and consuming them in typical food amounts poses no toxicity risk. However, excessive intake through high-potency supplements may lead to flavonoid toxicity. Vulnerable populations, such as the elderly with marginal iron deficiency, may face risks since flavonoids can bind nonheme iron (18). While most flavonoids are generally safe, excessive consumption has been associated with adverse effects, including gastrointestinal symptoms, hemolytic anemia, hepatotoxicity, and potential interactions with medications (19). Long-term use of flavonoids has also been linked to conditions such as contact dermatitis in sensitive individuals and estrogen-related effects in men and breast cancer (20).
The results indicated that none of the studied treatments caused a significant reduction in cancer cells compared to the control. Based on this finding, the use of B. monnieri under the applied treatments is not recommended for controlling the growth of skin cancer cells. However, B. monnieri has shown promising results in studies involving other cancer cell types. The anticancer effects of B. monnieri remain unproven at the clinical level in humans. Currently, no clinical evidence supports the potential anticancer activity of this plant. Reports indicate that the methanolic extract of B. monnieri contains phenolic bioactive molecules that act by inhibiting cell motility (4). Studies on certain cancer cell lines have revealed that B. monnieri initiates DNA fragmentation after treatment. The proposed mechanism of action involves bacoside A binding to the enzyme CaMK2A. This binding triggers phosphorylation, enabling CaMK2A to interact with ryanodine receptors on the endoplasmic reticulum membrane. This interaction increases calcium ion release, leading to significant fluid absorption via macropinocytosis.
Consequently, cells swell and eventually undergo lysis (4). Research indicates that the ethanolic extract of B. monnieri regulates endogenous oxidative marker levels in the brains of pre-pubescent mice (21). A study by Russo et al. investigated DNA damage and cytotoxicity caused by H₂O₂ in human non-immortalized fibroblast cells treated with ethanolic B. monnieri extract. Their findings revealed that the extract inhibits superoxide anion formation, demonstrating its free radical scavenging ability. It provides protective effects against H₂O₂-induced cytotoxicity and DNA damage (22).
Histological and animal studies have shown that B. monnieri extract enhances the system's defense against oxidative stress by reducing free radical formation in the brain (21). Bacosides in B. monnieri inhibit lipoxygenase activity, eliminate free radicals, and protect neurons in the prefrontal cortex, hippocampus, and striatum from cytotoxicity and DNA damage associated with Alzheimer's disease. Additionally, bacosides increase glutathione peroxidase activity, facilitate iron chelation, and promote nitric oxide-mediated cerebral vasodilation, resulting in improved memory performance (23).
It is believed that bacosides repair damaged neurons by enhancing kinase activity and neurosynthesis, restoring synaptic activity and improving nerve impulse transmission (24). Studies have demonstrated that oral administration of B. monnieri extract increases antioxidant levels such as SOD, GSH, and CAT, while reducing free radicals like LPO and NO. It also accelerates wound closure in mice due to its antioxidant properties (25). Furthermore, B. monnieri ethanol extract at 20 mg/kg body weight inhibits tumor progression by reducing LDH levels (26).
The extract of B. monnieri enhances autophagy, indicating a novel function that warrants further investigation (4).
The results showed that a concentration of 0.5 μg/mL 2IP significantly increased the survival of normal cells while having no notable effect on the survival of cancer cells. PGRs play a critical role in the accumulation of bioactive compounds. The type and concentration of auxins and cytokinins influence growth and product formation in callus and plant cell suspension cultures, with hormone concentration being a key factor in the accumulation of secondary metabolites such as phenolics and flavonoids (7). For example, research on Garcinia brasiliensis Mart. callus cultures demonstrated that treatment with 2,4-D and 2IP significantly increased total flavonoid levels (27). Similarly, studies on date palm suspension cultures found that a combination of 1 mg/L 2,4-D and 5 mg/L 2IP enhanced biomass accumulation, while treatment with 5 mg/L 2,4-D and 2.5 mg/L 2IP induced notably higher levels of phenolics and flavonoids (28). These findings highlight the importance of optimizing hormone concentrations to achieve balanced enhancement of both biomass and secondary metabolite production.
In the context of flavonoids, apigenin—a subtype of flavones—has demonstrated efficacy against various cancer types, including prostate cancer, colon cancer, breast cancer, and melanoma. Apigenin has been shown to inhibit metastasis, cell migration, invasion, and epithelial-mesenchymal transition (EMT) across these cancer models. Notably, it has been observed to reduce stem cell properties in breast cancer cells (29). This underscores the potential of flavonoid compounds in cancer treatment, further emphasizing the importance of PGR-optimized secondary metabolite production for therapeutic applications.
5.1. Conclusions
This study developed an efficient, effective, and reproducible protocol for the micropropagation of B. monnieri, which is at risk of extinction from its natural habitat. Additionally, the extract obtained from B. monnieri contained a high amount of secondary metabolites. This is the first report in which active compounds derived from tissue culture of B. monnieri were tested on different cell types. The use of hormones to increase flavonoid content in B. monnieri demonstrated that these hormones can significantly enhance flavonoid levels. However, the application of this plant under the mentioned treatments is not recommended for the treatment of skin cancer, and further research is necessary.
Regarding the effect of the studied treatments on normal cells, the results showed that a concentration of 0.5 μg/mL 2IP significantly increased the growth of fibroblast cells. Based on this finding, the use of this hormone in the tissue culture of the B. monnieri plant is recommended to enhance the viability of fibroblast cells for use in cosmetics and health products, although additional studies are required.