1. Background
Cardiovascular diseases can be directly linked to obesity, which is defined as an excessive accumulation of fat in the body (1). A diet rich in saturated fats, trans fats, or refined sugars is associated with increased production of inflammatory biomarkers, especially in patients with diabetes and those who are overweight. Adipose tissue has been shown in multiple studies to secrete adipokines and other inflammatory mediators, making it an endocrine organ (2).
Lipoprotein-associated phospholipase A (Lp-PLA2) is a member of the A2 phospholipase family (3). Lipoprotein-associated phospholipase A catalyzes oxidized phosphatidylcholine into oxidized non-esterified free fatty acids and lysophosphatidylcholine, which has a pro-inflammatory effect and plays a role in atherogenesis and subsequent cardiovascular disorders (4). High concentrations of cystatin C have been linked to increased mortality risk, making it a new risk factor for cardiovascular diseases (5). Cystatin C is a protein consisting of 122 amino acids with low molecular weight that inhibits the enzyme cysteine protease and is present in all nucleated cells of the body (6). Adipokines, secretory factors from fat tissue, can have either pro-inflammatory or anti-inflammatory effects. Adipose tissue dysfunction can cause irregular production of these adipokines. Cardiac failure can be caused by inflammation, which is one of the key factors in damaging cardiac cells (7). The accumulation of fat cells in blood vessels and plaque formation are linked to the inflammatory response (8).
Toll-like receptor (TLR) play a crucial role in activating innate immunity and regulating inflammation through a shared signaling pathway (9). Toll-like receptor can be categorized as cell surface or intracellular, depending on their location within the cell.
Cellular processes like translation, transcription, and regulation of apoptosis and inflammation are significantly influenced by protein kinase R (PKR) (10). Indeed, pro-inflammatory cytokines such as TNF-α, IL-1, and IFN-γ activate PKR, which then triggers inflammation-related pathways. Protein kinase R acts as an activator in signaling cascades related to stress and inflammation-induced protein kinase activity (11). Protein kinase R’s role in the activation of IκB kinase depends on the stimulus, either catalytic or constitutive. Protein kinase R regulates the activities of stress-activated protein kinases, including p38 and JNK (C-Jun NH2 terminal kinase), in a pathway that results in pro-inflammatory cytokine production (12). Individuals who engage in regular physical activity have a reduced risk of developing cardiovascular diseases and other chronic conditions associated with aging. New research shows that exercise reduces levels of certain anti-inflammatory cytokines and chemokines in the body. Scientific evidence indicates that regular aerobic exercise decreases inflammatory factors (13).
2. Methods
2.1. Animals
For this study, we acquired 24 male Wistar rats from the Laboratory Animal Breeding Center of Jundishapur University of Medical Sciences in Ahvaz, Iran. The research adhered to ethical guidelines for working with animals, as outlined in the Helsinki protocol and was approved by the Islamic Azad University of Ahvaz branch (ethics code: IR.IAU.AHVAZ.REC.1402.018). The obesity model group received a high-fat diet (HFD) comprising sixty percent of their standard rodent feed purchased from the Isfahan Royan Biotech Company. To create the high-fat pellets, 45 kg of standard pellet powder was combined with 30 kg of animal fat, resulting in 100 kg of high-fat pellets with a 60% fat content. For the high fructose diet, 250 mL of fructose liquid was dissolved in 750 mL of water to make a 25% solution. Each day, a fresh 25% fructose solution was prepared and provided to the rats in 500 mL bottles. The control diet included ingredients such as casein, cornstarch, sucrose, a mixture of vegetable and animal fats, maltodextrin, soybean oil, calcium carbonate, monocalcium phosphate, corn gluten, corn, wheat bran, a mineral premix, and various vitamins.
2.2. Aerobic Exercise Protocol
The 6-week training protocol consisted of moderate-intensity exercises. To achieve this, the overweight participants underwent treadmill exercise five times a week for six weeks. The training sessions were conducted at the end of the animals' sleep cycle, between 16:00 and 18:00 pm. The speed and duration of the treadmill exercise gradually increased: Starting at 10 m/min for 10 minutes in the first week, 10 m/min for 20 minutes in the second week, 14 to 15 m/min for 20 minutes in the third week, 14 to 15 m/min for 30 minutes in the fourth week, and 17 to 18 m/min for 30 minutes in the fifth through eighth weeks. To ensure consistent adaptations, the training variables remained unchanged during the final week.
2.3. Evaluation of Neutrophil/Lymphocyte Ratio
To calculate the neutrophil/lymphocyte ratio, the rats were anesthetized using a combination of ketamine (90 mg/kg) and xylazine (90 mg/kg) (14). Following anesthesia, the chest was opened, and a blood sample was collected from the heart. The laboratory then performed cell counting using the Sysmex model N-21KX machine and the Giemsa staining protocol. The neutrophil/lymphocyte ratio was calculated using an optical microscope, based on the percentage of neutrophils and lymphocytes.
2.4. Evaluation of Lipoprotein-Associated Phospholipase A
The rats were anesthetized with a mixture of Ketamine and xylazine for the Lp-PLA2 measurement. Afterward, the chest was opened, and a blood sample was collected from the heart. The Lp-PLA2 concentration was then determined using a specific kit and the ELISA methodology, following the kit's instructions (15).
2.5. Evaluation of Cystatin C
The rats were given a combination of Ketamine and Xylazine to anesthetize them for cystatin C measurement. Next, they opened the chest and gathered a blood sample from the heart. Subsequently, the cystatin C concentration level was assessed by means of a special kit and the ELISA method, in accordance with the kit’s instructions (16).
2.6. Real time-PCR Genes Expression of Protein Kinase R/Toll-Like Receptor
Gene expression was assessed through SYBR green-based quantitative PCR (q-PCR) utilizing a commercial PCR system. The DNA purified via complementary DNA (cDNA) was amplified with the primers detailed in Table 1. The PCR protocol involved an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles consisting of denaturation at 95°C for 15 seconds and annealing and extension at 60°C for 60 seconds. This was succeeded by a dissociation phase that included 15 seconds at 95°C, 60 seconds at 60°C, another 15 seconds at 95°C, and a final 15 seconds at 60°C. The specificity of the products was verified through a SYBR green single melting curve analysis and the presence of a single, correctly sized product on a 1.2% agarose gel. Each sample was analyzed in duplicate, with the cDNA values normalized to the Ct value of GAPDH from the same sample normalized to the Ct value of the input sample. Relative fold changes were determined by designating the mean fraction of normal samples (17). Rat PKR and TLR mRNA levels were quantified using a PCR array plate, and further validated by qPCR with gene and species-specific primers.
| Gene | Forward | Reverse |
|---|---|---|
| PKR | 5′-AAGGTGAACGTGGATGAAGTT-3′ | 5′-AGCATCAGGAGTGGACAGAT-3′ |
| TLR | 5′- TATCAAGGTTACAAGACAGGTTTAAG-3′ | 5′-GCCTAAGGGTGGGAAAATAGAC-3′ |
| GAPDH | 5′-CGGAATTCTACCCTTGGACCCAGAG-3′ | 5′-CCCAAGCTTCGATCCTGAGACTTCCAC-3′ |
The Sequences of Primers
2.7. Statistical Methods
SPSS software was used for data analysis. The ANOVA test and post hoc test were employed to compare the groups. The data were analyzed and presented as Mean ± SEM, with significance set at a P-value of less than 0.05.
4. Results
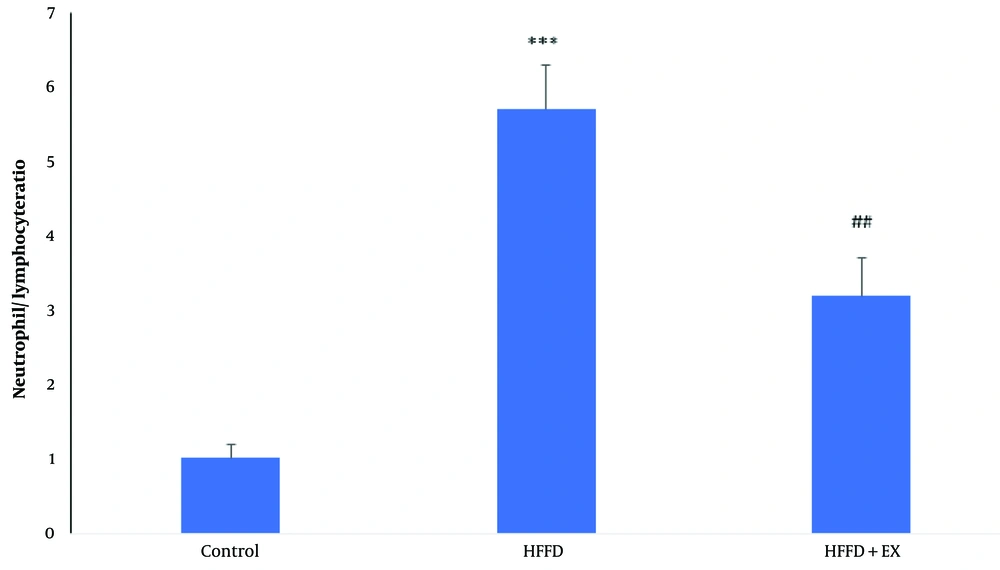
4.1. Exercise Improved Neutrophil/Lymphocyte Ratio in Obese Rats
As it shows in Figure 1, obesity caused to significant increases (P ≤ 0.001) in neutrophil/lymphocyte counting ratio compare to control group. Although six weeks-exercise caused to significant decreases (P ≤ 0. 01) of neutrophil/lymphocyte counting compared to the obese rats.
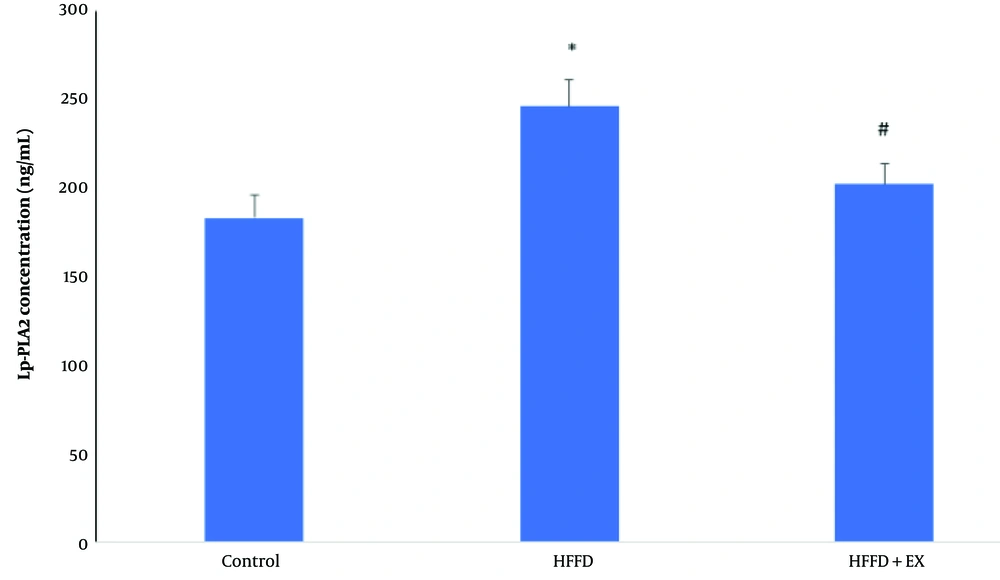
4.2. Exercise Improved Lipoprotein-Associated Phospholipase A in Obese Rats
As shown in Figure 2, obesity caused a significant increase in Lp-PLA2 concentration levels in the blood compared to the control group (P ≤ 0.05). However, the six-week exercise regimen led to a significant decrease in Lp-PLA2 concentration levels compared to the obese rats (P ≤ 0.05).
Comparison of lipoprotein-associated phospholipase A (Lp-PLA2) concentration level in different groups, including: Control, HFFD (high fat/fructose diet) and HFFD + EX (high fat/fructose diet plus exercise). The comparison of groups has been done by one-way ANOVA, and HSD. * vs. control and # vs. HFFD group.
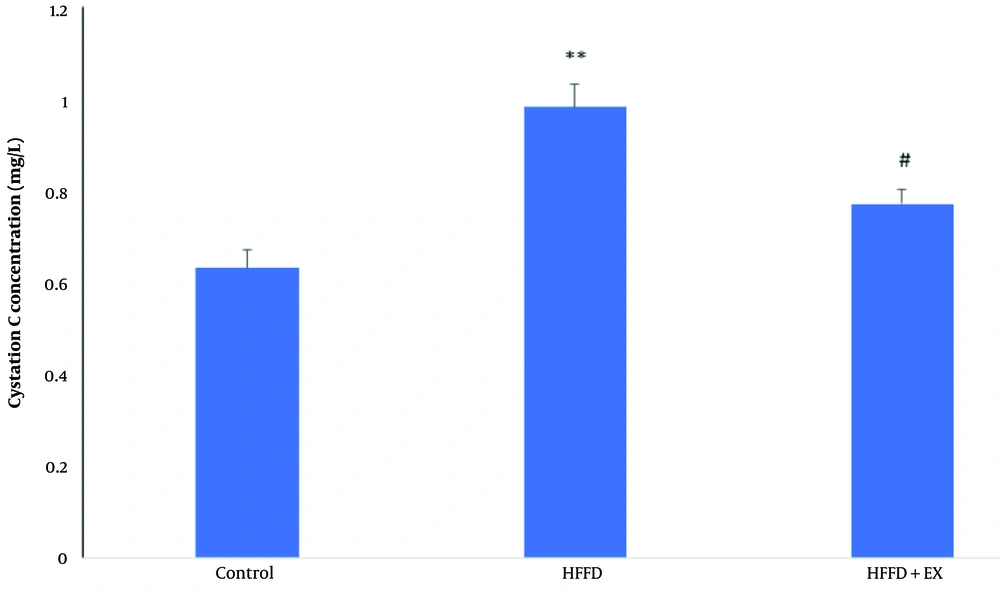
4.3. Exercise Improved Cystatin C in Obese Rats
As shown in Figure 3, obesity caused a significant increase in cystatin C concentration levels (P ≤ 0.01) in the blood compared to the control group. However, the six-week exercise regimen led to a significant decrease in cystatin C concentration levels compared to the obese rats (P ≤ 0.05).
4.4. Exercise Improved Protein Kinase R/Toll-Like Receptor Pathway in Obese Rats
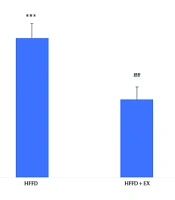
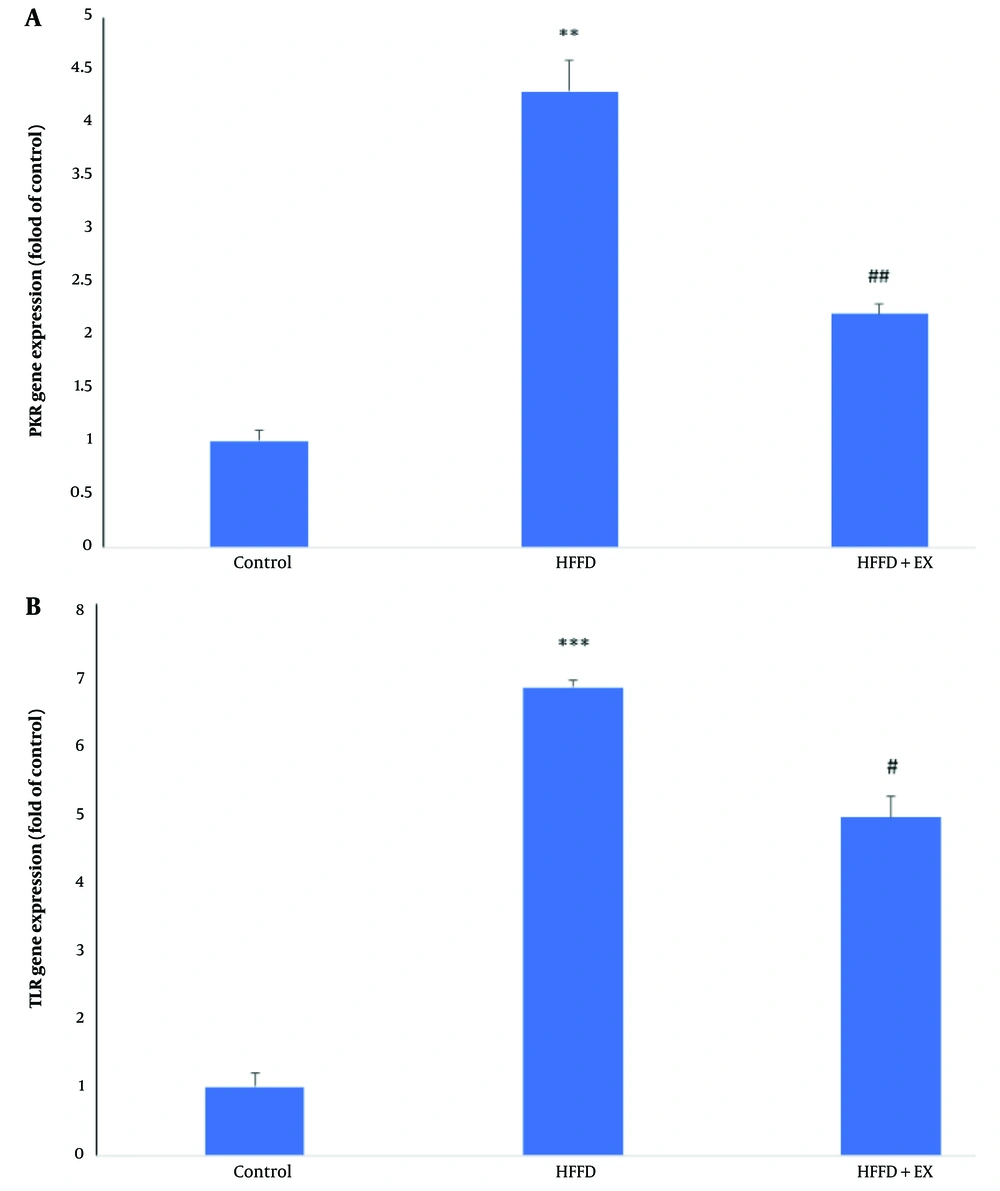
As shown in Figure 4A, obesity caused significant increases in PKR gene expression levels (P ≤ 0.01) in cardiac tissue compared to the control group. However, six weeks of exercise resulted in significant decreases in PKR gene expression levels compared to the obese rats (P ≤ 0.01). Additionally, obesity caused significant increases in TLR gene expression levels (P ≤ 0.001) in cardiac tissue compared to the control group (Figure 4B). However, six weeks of exercise led to significant decreases in TLR gene expression levels compared to the obese rats (P ≤ 0.05).
Comparison of gene expression level of protein kinase R (PKR) (A) and TLR (B) in different groups, including: Control, high fat/fructose diet (HFFD) and HFFD + exercise (EX). The comparison of groups has been done by one-way ANOVA, and HSD. ** and *** vs. control and # and ## vs. HFFD group.
5. Discussion
The current research findings show a noteworthy distinction in cystatin C, Lp-PLA2 concentration, and the neutrophil/lymphocyte ratio between the normal and obese groups. This suggests that consuming high-fat food with high fructose content induces inflammatory disorders in obese rats. Moreover, the high-fat/fructose diet led to a significant increase in TLR and PKR gene expression compared to the control group. The aforementioned inflammatory biomarkers showed improvement in the obese rats after 6 weeks of exercise.
Obesity, insulin resistance, and fatty liver disease are risks associated with the excessive consumption of a HFD and fructose. Fructose is a prevalent monosaccharide found abundantly in the human diet (15). However, unlike glucose, which is metabolized throughout the body, fructose is primarily metabolized in the liver. The presence of fructose affects weight control (16). When fat is combined with fructose, it leads to greater fat storage and weight gain compared to when either fat or fructose is consumed separately. The blocking of leptin signaling causes a preference for high-fat foods, resulting in rapid weight acquisition and fat accumulation (17).
A diet high in fat and fructose increases oxidative stress levels. Mitochondrial oxidative stress hinders both mitochondrial β-fatty acid oxidation (through enol-CoA hydratase inhibition) and the tricarboxylic acid cycle (by blocking aconitase), resulting in reduced ATP production via oxidative phosphorylation (17). The main effect is the inhibition of AMP-activated kinase (AMPK), a key player in ATP regeneration during times of low energy. By metabolizing fructose, the KHK-C pathway lowers ATP levels within cells (18). Leptin resistance is not caused by HFDs but by ingested or endogenous fructose. Leptin resistance is affected by fructose, but weight gain is worsened by high-fat and energy-dense diets in animals with leptin resistance (19).
The cardiac tissue of rats shows significant changes in the expression of the PKR gene after six weeks of aerobic exercise with a high-fat/fructose diet. The PKR or EIF2AK2 gene, a serine-threonine kinase, is a crucial player in cellular processes like translation, transcription control, apoptosis regulation, and inflammation (20). Interferons, viral infection, high cholesterol diet, cytokines, pathogens, radiation, heme limitation, ER stress, and mechanical stress activate signal transduction (21). Due to its catalytic activity, PKR can directly couple with the metabolic pathway and play a role in pathogen recognition. In virus-infected cells, PKR is activated and inhibits protein synthesis and apoptosis through phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α), which induces an immune response (22). Activation of PKR, a protein kinase, occurs when there is double-stranded RNA induced by viral interferon and pro-inflammatory cytokines such as TNF-α, IL-1, and IFN-γ (23). Protein kinase R’s functions are determined by the proteins involved in signaling. Activated PKR influences various pathways involving transcription factors like IRF3, NF-κB, c-Jun, and ATF2, regulating inflammation and immunity (24).
Furthermore, the findings from the present research demonstrate a notable variation in TLR gene expression within the cardiac tissue of rats across all groups. The TLR gene expression level was significantly elevated in the high-fat/fructose diet group compared to the control rats. In contrast, there was a statistically significant decrease in TLR gene expression in the obese rats that underwent a high-fat/fructose diet plus aerobic exercise.
Toll-like receptor are found on the plasma membrane of immune cells like macrophages and dendritic cells. They play a crucial role in activating innate immunity and regulating inflammation (25). Toll-like receptor activation leads to signaling cascades that induce the production of proinflammatory cytokines and type I interferons. The increases in cytokines cause fever, pain, and inflammation, while the interferons mediate antiviral responses.Toll-like receptors operate through two signaling pathways: The adapter protein TRIF activates transcription factors IRF3 and IRF7, which induce the cellular immune response caused by the virus, while the other pathway involves the adapter protein MyD88, which stimulates transcription factor NF-κB and activates AP-1, leading to the expression of inflammatory cytokines (26). The TLR homodimer complexes with two MD-2 receptor cores in the form of two copies of TLR4-MD-2. They then bind to ligands such as LPS to generate two copies of the TLR4-MD-2-LPS complex. When this complex appears on the cell membrane, the MyD88-dependent signaling pathway is activated (27). The early-phase expression of NF-κB and the production of inflammatory cytokines occur through this signaling pathway. Interactions between cytoplasmic proteins MyD88 and TIRAP and TLR4 receptors happen when ligands bind to the receptors (28). MyD88 and TIRAP, in turn, form a signaling cascade that includes the IRAK4 kinase, activates IRAK1, and, as a result of binding these two kinases, activates TRAF6. This then activates TAK1, followed by IKKα (IKK1) and IKKβ (IKK2), leading to the formation of a trimer with p50 and p63 proteins, activating NF-κB. The phosphorylated and active NF-κB protein enters the cell nucleus, causing the expression of pro-inflammatory cytokine genes such as TNFα and IL-6 (29). TAK1 activates INK and triggers the AP1 protein, which leads to pro-inflammatory cytokine production in the nucleus. Studies have indicated that exercise can influence the TLR receptor, which plays a role in multiple cardiovascular diseases (30). Toll-like receptor plays a crucial role in inflammatory and apoptotic processes in cardiac tissue, contributing to the development of cardiovascular diseases.
In summary, the findings of this research showed that inducing obesity in rats with a high-fat/fructose diet increases the expression of PKR and TLR genes in the cardiac tissue, which was consistent with significant increases in cystatin C, Lp-PLA2 concentration levels, and the neutrophil/lymphocyte ratio. Also, the results showed that aerobic exercise reduces the expression of PKR and TLR genes in the cardiac tissue of obese rats, which was associated with a decrease in inflammatory biomarkers. Therefore, it is suggested that aerobic exercise can be considered a preventive and treatment strategy for cardiovascular problems in obese patients.