1. Background
A common belief in the scientific and dairy communities is the inverse relationship between milk production and the reproductive performance of dairy cows (1). Low fertility in dairy cows remains a major challenge for the industry (2). However, advances in reproductive management and genetics have significantly improved reproductive performance (3). Progesterone deficiency has been associated with reduced pregnancy rates per artificial insemination (P/AI), abnormal early embryo development, and decreased signaling for maternal pregnancy detection (4). Numerous studies have aimed to increase blood progesterone levels to improve P/AI in dairy cows (5, 6).
Gonadotropin-releasing hormone (GnRH) is a decapeptide produced and released by neurons in the hypothalamus (7). It plays a central role in regulating female reproductive functions via the hypothalamic-pituitary-ovarian axis (8). Gonadotropin-releasing hormone is commonly used to stimulate the pituitary gland to release gonadotropins, including luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby influencing ovarian responses (9, 10). As a result, GnRH analogs are widely used in bovine reproductive programs to induce an LH surge and trigger ovulation, particularly in timed artificial insemination (TAI) protocols (11, 12).
Administration of GnRH agonists at the beginning of diestrus (days 4 - 9) after AI promotes sustained luteinization of follicles and may increase embryo survival rates (13). The GnRH's role in delaying luteolysis and affecting follicular dynamics positively contributes to an increased fertilization rate (14). Various doses of GnRH used in artificial insemination (AI) have been reported to improve conception rates in crossbred dairy cows, with no significant differences between different GnRH doses (15).
In contrast, several studies have indicated that administering GnRH during estrus and AI does not enhance fertility rates in dairy cows (16, 17). Previous research has shown that GnRH-based TAI protocols in Bos indicus beef cattle often result in unfavorable P/AI outcomes (18, 19). This has been attributed to ovulatory failure and the lack of a new follicular wave at the start of the protocol (20). Several factors influence the ovulatory response to the initial GnRH treatment in TAI protocols, including the presence or absence of a dominant follicle with ovulatory potential, the stage of the estrous cycle, and the overall cyclic status (21). Additionally, circulating progesterone levels, whether physiological or influenced by external manipulation, can suppress the GnRH-induced LH surge and interfere with ovulation (22).
These studies have not reached a clear consensus on the therapeutic benefits of GnRH treatments. While some research indicates a positive effect of post-AI GnRH on P/AI, other studies have found no benefit or even a decrease in P/AI (23). These results suggest the need for further research to identify cow- or farm-level factors that influence the effectiveness of using GnRH at the time of AI to improve fertility in dairy cows.
2. Objectives
This study aimed to evaluate the influence of GnRH treatment on superovulation response and embryo retrieval in Holstein heifers. For this purpose, 21 Holstein heifers were inseminated twice: Once at the onset of standing estrus and again 12 hours later. In the treatment group (13 heifers), a single dose of GnRH was administered at the same time as the second insemination. The superovulation response was assessed based on the number of corpora lutea (CL), unovulated follicles (UoF), total recovered embryos/ova, and the number of transferable embryos.
3. Methods
The experiment was conducted at the Zagros Milk and Meat Complex in Shahrekord city (32°19′32″N 50°51′52″E), Iran, from September 2023 to February 2024. The average annual daily temperature was 12.4 ± 2.38°C (Min: -8.4°C, Max: 33.9°C), and the annual daily relative humidity was 36.4 ± 4.9%. The animals were fed a balanced ration based on their physiological state to ensure adequate intake of energy, protein, and minerals (NRC). Water was provided ad libitum. The farm housed 2,537 lactating cows with an average daily milk yield of 39.8 kg, a pregnancy rate of 45.7%, and an average of 191 days in milk. The experimental procedures were conducted in accordance with the guidelines of the university's ethical committee on animal research.
3.1. Superovulation Procedure
A total of 21 Holstein heifers (age: 135.15 months; weight: 361.05 kg) were selected based on general health status and ovarian function. Heifers with ovarian abnormalities, such as cystic follicles or static ovaries, as well as any uterine abnormalities, were excluded. All heifers received two consecutive doses of prostaglandin F2α (PGF2α). Ten days after standing estrus, the heifers were administered descending doses of 975 IU hr-FSH (Cinal-F, Cinagen, Iran) every 12 hours, as follows: 225, 225, 150, 150, 75, 75, 37.5, and 37.5 IU for 8 consecutive intramuscular injections. Two PGF2α doses were administered concurrently with the 5th and 6th FSH injections. At the time of the second PGF2α administration, the heifers were divided into two groups: The control group (n = 8), which did not receive any additional treatment, and the GnRH group (n = 13), which received a single 25 µg dose of GnRH (Gonadorelin, Aburaihan, Iran). The heifers were inseminated twice: Once at standing estrus and again 12 hours later. On day 7 after standing estrus, the heifers were prepared for uterine flushing.
3.2. Uterine Flushing and Embryo Recovery
Non-surgical uterine flushing was performed using an 18-inch Foley catheter, two-way silicone tubing, and an embryo collection filter. The flushing medium consisted of Dulbecco’s phosphate-buffered saline supplemented with 0.5 g of bovine serum albumin, 1000 IU penicillin, and 1 g streptomycin per liter (pH: 7.2 - 7.4; osmolality: 290 - 310 mOsm). The uterus was flushed with 700 - 1200 mL of the flushing medium. After flushing, all heifers received two consecutive doses of PGF2α at 12-hour intervals.
The recovered fluids were examined for ova/embryos in a 10 cm Petri dish under a stereomicroscope. The embryos were evaluated according to international embryo transfer guidelines, assessing the developmental stage and homogeneity of blastomeres and embryos.
3.3. Statistical Analysis
The superovulatory response was evaluated based on the number of CL, UoF, total recovered embryos/ova, and the number of transferable embryos. Statistical analysis was conducted using logistic regression with the GENMOD procedure in SAS. A P-value of less than 0.05 was considered statistically significant.
4. Results
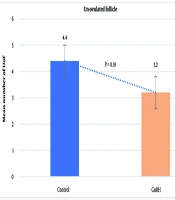
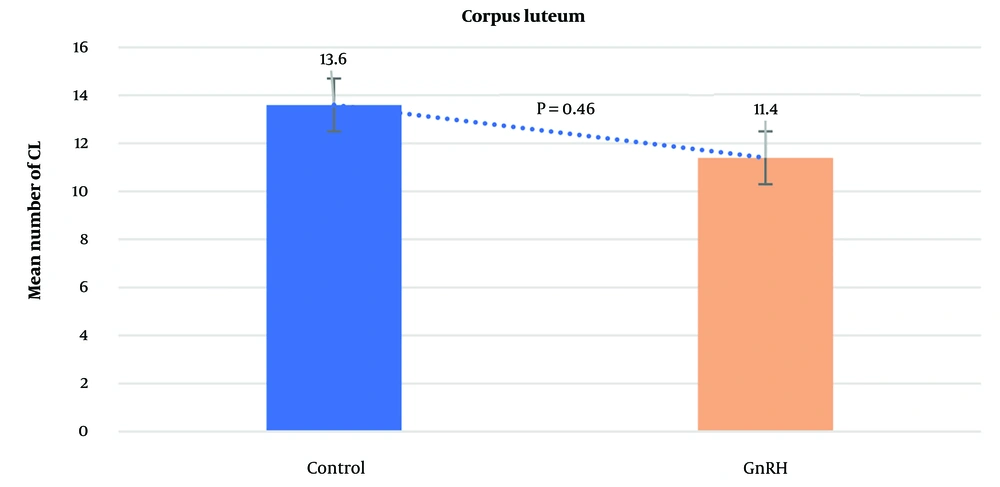
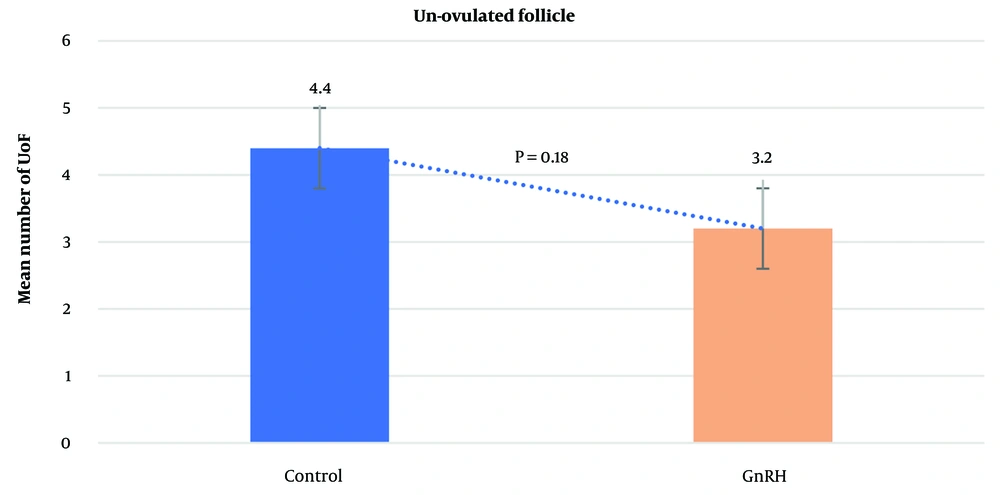
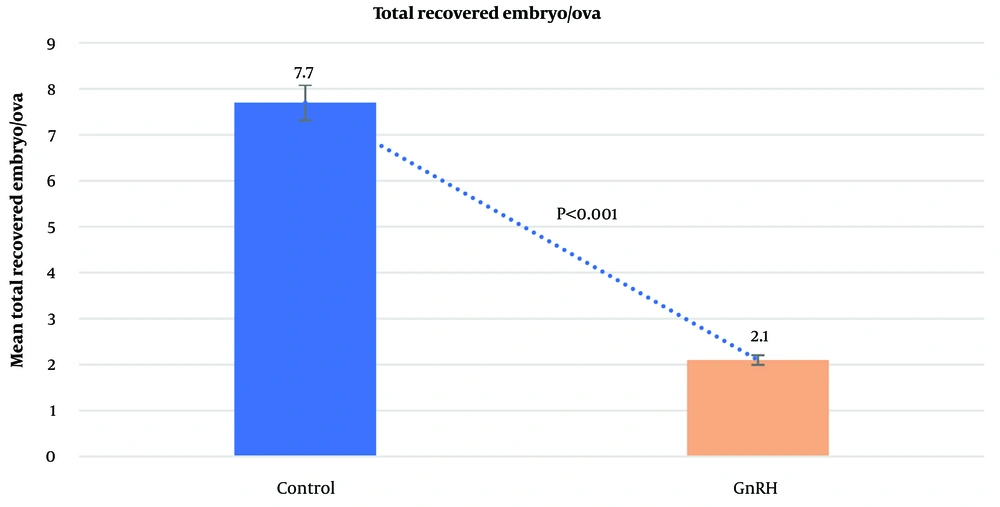
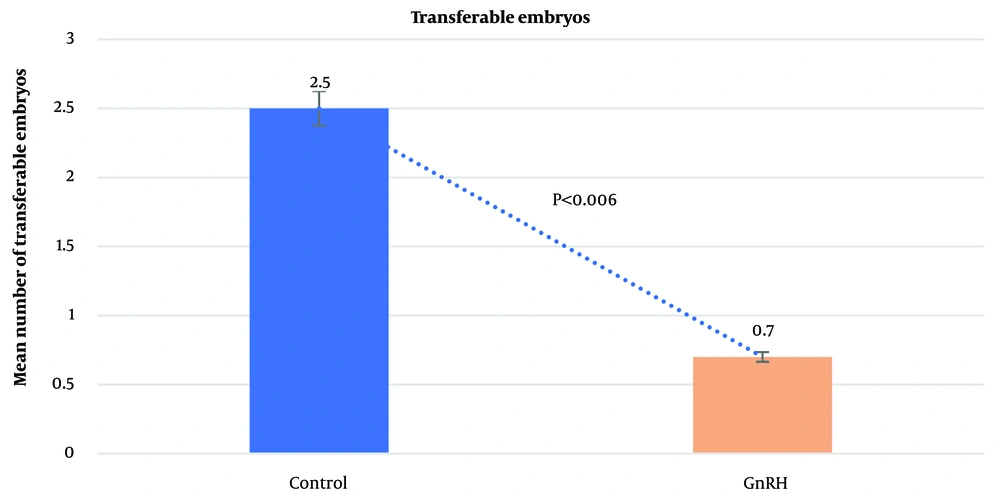
The average number of CL did not differ significantly between the control group (13.6 ± 0.53) and the GnRH group (11.4 ± 0.92; P = 0.4612). Similarly, the mean number of UoF was not significantly different between the groups (GnRH: 3.2 ± 0.47; control: 4.4 ± 0.56; P = 0.1853). However, the mean total number of recovered embryos/ova was significantly higher in the control group (7.7 ± 2.01) compared to the GnRH group (2.1 ± 1.14; P < 0.0001). The average number of transferable embryos was 2.5 ± 0.43 in the control group and 0.7 ± 0.44 in the GnRH group (P < 0.0062). The general data obtained from the study are presented in Figures 1 - 4.
5. Discussion
At the end of the superovulation program, the administration of GnRH did not enhance the superovulation response. In this study, the use of GnRH during superovulation did not affect the average number of CL or UoF but resulted in a decrease in the total number of recovered embryos/ova and transferable embryos in heifers. These results suggest that GnRH may not disrupt ovulation itself but instead affects the transfer of oocytes from the ovary to the uterus, likely by influencing the biology of the oviduct. The oviduct requires a precise balance of estradiol and estrogen, along with the effects of oxytocin, to regulate peristaltic movements. GnRH likely disturbed this estradiol-progesterone balance, rendering the oviduct ineffective. If the oviduct environment is unfavorable, released oocytes may not enter the uterus.
The findings of Mohammadi et al. (17) showed that GnRH administration to primiparous and multiparous dairy cows in cold and moderate weather conditions (without heat stress) did not affect pregnancy rates per artificial insemination (P/AI) or pregnancy survival rates. Gonadotropin-releasing hormone administration has been reported to double the probability of an additional CL 11 days after insemination (24). Besbaci et al. (25) conducted a meta-analysis of 107 studies to assess the impact of GnRH treatment on P/AI in cattle. The analysis revealed that GnRH treatment improved P/AI in cows with very poor (< 30%) and poor fertility (30.1 - 45%) but showed no significant benefit for cows with very good fertility (> 60.1%). The discrepancy between these studies and the present study's findings may be due to differences in the timing of GnRH administration and the timing of CL and ovary evaluation.
One study indicated that superovulation protocols induced by GnRH can increase luteinizing hormone/choriogonadotropin receptor expression before embryo implantation, potentially impairing ovarian function (26). It is well-established that low pregnancy rates following superovulation are associated with abnormal estrogen and progesterone levels (27). Li et al. (28) found that GnRH-stimulated superovulation protocols consistently showed high estrogen and low progesterone levels, with an increase in the expression of key enzyme genes involved in progesterone synthesis and a decrease in genes responsible for converting progesterone to estrogen. These alterations may be due to negative feedback mechanisms triggered by abnormal hormone levels. In contrast, ovulation stimulated by human chorionic gonadotropin (hCG) resulted in elevated levels of both estrogen and progesterone (29). However, GnRH-induced ovulation was associated with reduced progesterone levels during the preimplantation phase (28).
Research indicates that the duration of LH stimulation induced by GnRH is shorter than that observed in the physiological state, which may result in inadequate luteal function and potentially premature dissolution of the CL (30). In mouse models, ovulation stimulated by GnRH led to smaller embryos and placentas compared to those from natural mating, with a significantly higher rate of embryo resorption in GnRH-stimulated mice than in naturally mated mice (26). Similarly, GnRH stimulation in rabbits resulted in high abortion rates and persistently low progesterone levels (31). In llamas, administering GnRH on day 7 post-breeding did not alter progesterone concentrations compared to individuals with a single CL (32). These findings suggest that GnRH-induced ovulation may be associated with insufficient luteal function. Although the effects of GnRH on postovulatory progesterone levels varied across studies (26), estrogen expression consistently remained high. This evidence implies that GnRH-stimulated ovulation might disrupt normal hormone synthesis and gonadotropin receptor expression in the ovary, potentially impairing embryo implantation.
Numerous studies (33, 34) have demonstrated that superovulation results in the development of a large number of ovulatory follicles with a high ovulation rate (50 - 70%). However, the embryo/CL recovery rate remains very low (13 - 35%). This discrepancy suggests that poor embryo outcomes in superstimulated cows are not due to a reduced capacity for follicular growth and ovulation, which is typically constrained by the limited number of primordial follicles in the species (35). Even after flushing both the oviducts and uteri, the ratio of recovered ova to CLs in superovulated buffaloes remained low across various days post-AI (33). This suggests that superovulated cows may experience a failure in the oviduct fimbria's ability to capture the ovum.
A critical factor may be the binding of newly ovulated cumulus-oocyte complexes (COCs) to the ciliary tips of the epithelial cells lining the distal portion of the oviduct. The degree of adhesion affects the COC's ability to penetrate the oviduct lumen, where it encounters sperm migrating up the oviduct, influencing subsequent transport and compaction (36). In vitro studies (37) have shown that inadequate extracellular matrix formation, due to improper cumulus expansion, can reduce the cohesiveness between oviduct cilia and COCs, making it more difficult for COCs to enter the infundibulum. It is hypothesized that in superovulated cows, the interaction between the ovum and the ciliated cells of the endosalpinx may be disrupted by imbalanced steroid hormone levels (38). Additionally, the oviductal vascular endothelial growth factor system, which regulates oviductal contraction-relaxation and gamete/embryo transport, plays an active role during the peri-ovulatory period (39).
These findings suggest that GnRH may negatively impact embryo implantation by causing an excessive uterine response to elevated estrogen levels, which can disrupt gene expression related to endometrial remodeling, ion transport, and immune activity (28). In mammals, the function of the Fallopian tubes is significantly regulated by estrogen and progesterone, with tissue structure undergoing cyclical changes in alignment with hormonal fluctuations throughout the menstrual cycle. Estrogen acts through its receptors, ESR1 and ESR2, with ESR1 present in all Fallopian tube layers and ESR2 in select epithelial cells (40). Estrogen promotes epithelial cell growth, stimulates protein secretion from secretory cells, and enhances ciliary activity (41). Research shows that proper estrogen signaling in the oviduct's epithelial cells is crucial for embryo survival in mice (42). However, the absence of ESR1 in ciliated epithelial cells has only a minor effect on female fertility (41). Juengel et al. (43) linked reduced fertility in ewes to possible functional variations in the oviduct's isthmus among different animals.
Further research is needed to explore differences in immune-related gene expression in cumulus cells and their interactions with immune-related gene expression in the oviduct. This could help determine whether reduced immune-related gene expression is a key factor in the increased fertilization failures observed (44). Studies in sheep and cattle have shown a positive correlation between higher progesterone levels and increased embryo survival, both in peripheral and local circulation (45). However, this relationship may be quadratic, as excessive progesterone can also negatively affect embryo health (46). On day 3, some gene expressions were negatively correlated with progesterone levels. Specifically, negative correlations between the expression of TGFBR1, TGFBR2, and CSF2RB and progesterone levels suggest that progesterone helps to reduce inflammatory responses following mating. Overall, progesterone appears to be essential for creating a favorable oviductal environment for fertilization and early embryo development. Additionally, the embryo can influence gene expression in oviductal cells (47). Therefore, variations in ovum or embryo quality that affect embryo secretions or interactions with the oviduct could also impact gene expression in the oviduct (43).
Assessing the ovarian response is a key indicator of superovulatory success or failure. However, analyzing concurrent hormone profiles could provide further insights into the effects of GnRH administration on embryo recovery.
5.1. Conclusions
In general, the results of this study showed that GnRH, when used in a superovulation program, disrupted the mechanism of oocyte transfer from the ovary to the uterus, leading to a decrease in the total number of embryos and transferable embryos in heifers. Therefore, future efforts to mitigate the negative impact of superovulation on pregnancy establishment should focus on strategies to enhance ovarian function and shorten the period of elevated estrogen levels.